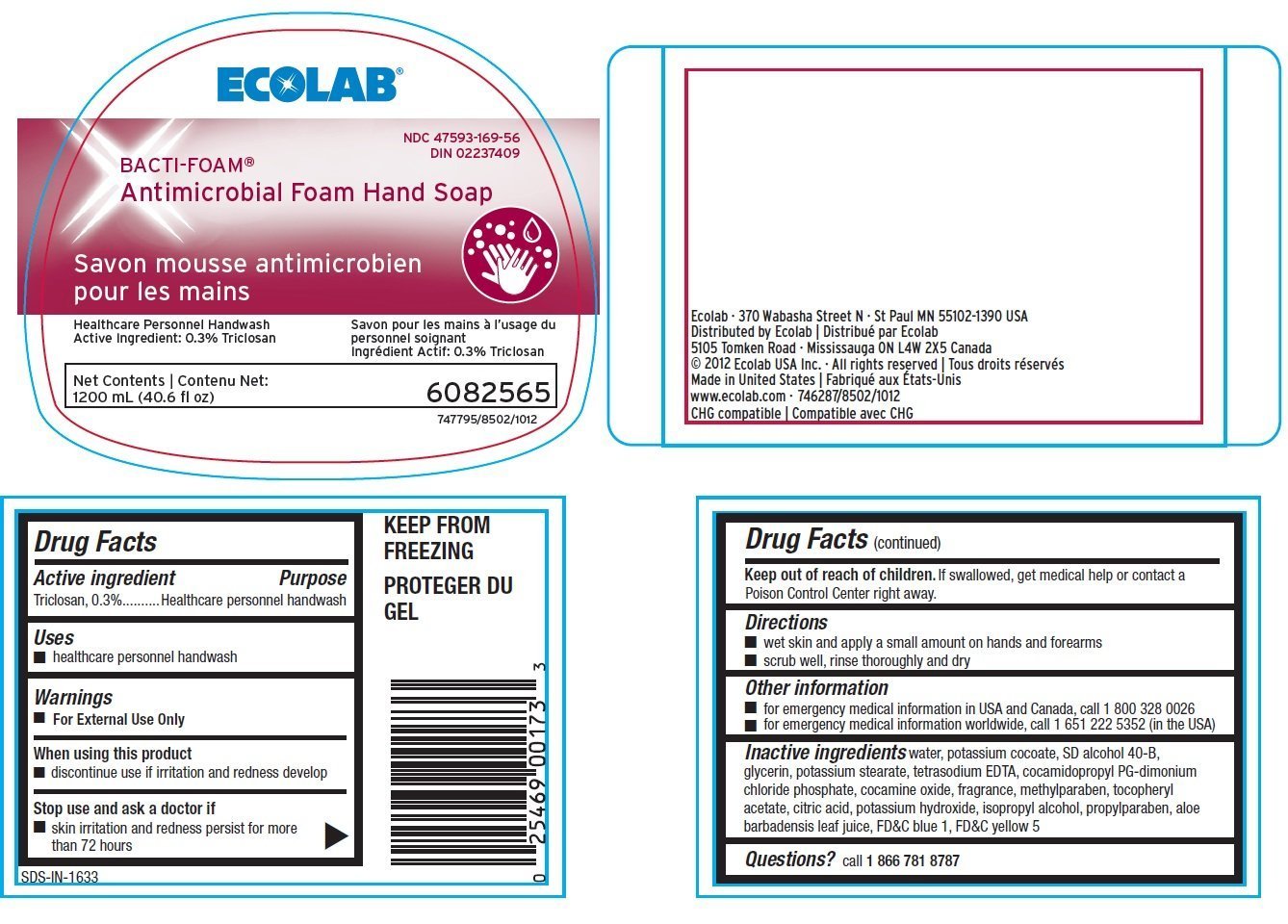
Bacti-Foam
Dosage form: solution
Ingredients: Triclosan 3mg in 1mL
Labeler: Ecolab Inc.
NDC code: 47593-169
Medically reviewed by Drugs.com. Last updated on Oct 9, 2023.
Triclosan, 0.3%
Healthcare personnel handwash
- healthcare personnel handwash
- For external use only
- discontinue use if irritation and redness develop
- skin irritation and redness persist for more than 72 hours
Keep out of reach of children. If swallowed get medical help or contact a Poison Control Center right away.
- wet skin and apply a small amount on hands and forearms
- scrub well, rinse thoroughly and dry
- for emergency medical information in USA and Canada, call 1.800.328.0026
- for emergency medical information worldwide, cal 1.651.222.5352 (in the USA)
Inactive ingredients water, potassium cocoate, SD alcohol 40-B, glycerin, potassium stearate, tetrasodium EDTA, cocamidopropyl PG-dimonium chloride phosphate, cocamine oxide, fragrance, methylparaben, tocopheryl acetate, citric acid, potassium hydroxide, isopropyl alcohol, propylparaben, aloe barbadensis leaf juice, FDC blue 1, FDC yellow 5
Questions? call 1.866.781.8787
| BACTI-FOAM
triclosan solution |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - Ecolab Inc. (006154611) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.