Skyrizi vs Humira for plaque psoriasis - How do they compare?
In a head-to-head clinical study between Skyrizi and Humira, researchers found that Skyrizi was better than Humira at clearing moderate-to-severe plaque psoriasis.
- In this study at 16 weeks, 72% of patients given Skyrizi achieved 90% clearer skin compared to 47% of patients given Humira, a statistically significant outcome.
- At 16 weeks, 109 patients using Humira who did not achieve 50% to 90% skin clearance were either switched to Skyrizi for a total of 44 weeks or stayed on Humira.
- In patients who switched to Skyrizi during the study, three times more people achieved 90% clearer skin when compared to Humira. Overall, 66% of those receiving Skyrizi who had formerly used Humira achieved 90% skin clearance at 44 weeks, compared to 21% of those who remained on Humira.
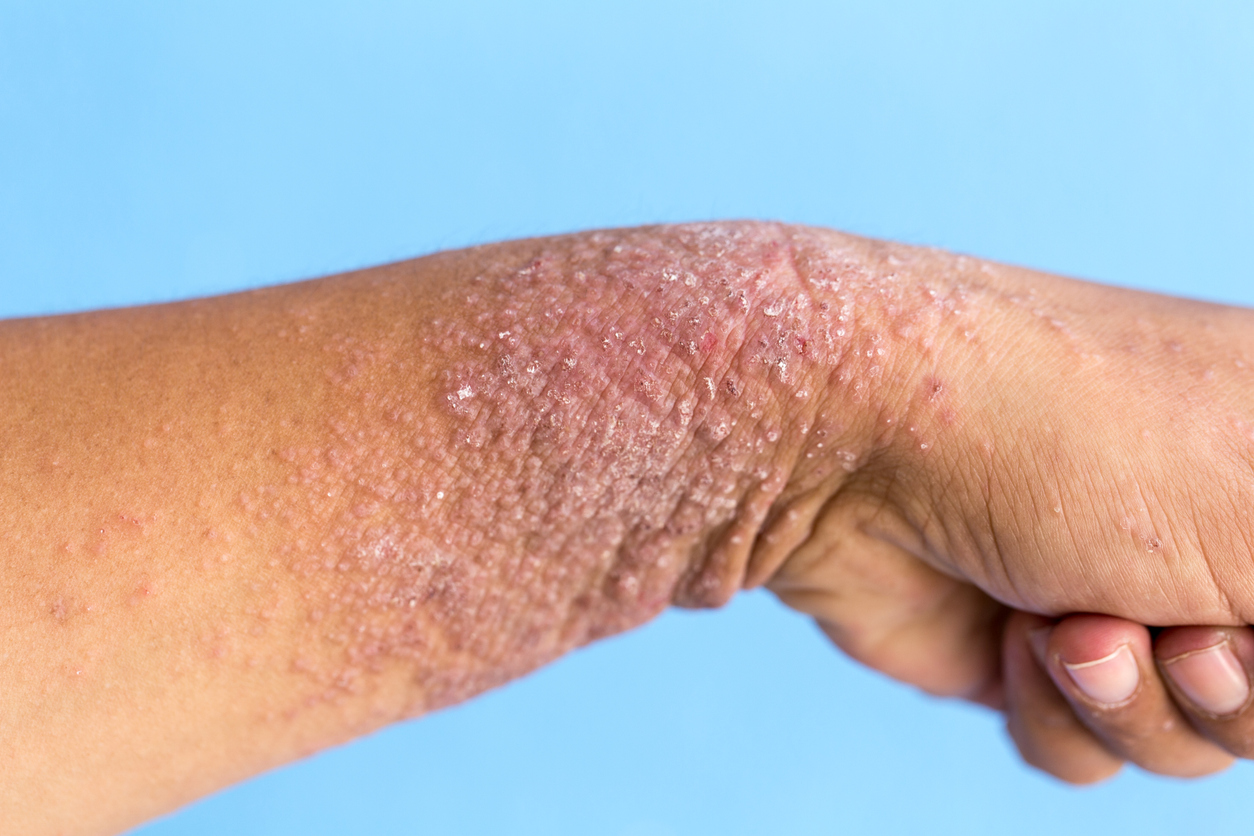
There were no new safety concerns in patients who switched from Humira to Skyrizi.
What are Skyrizi and Humira used for and how do they work?
Skyrizi was first approved by the FDA in 2019 to treat plaque psoriasis in adults. It is also approved to treat psoriatic arthritis and Crohn's disease in adults.
- Skyrizi works by selectively inhibiting interleukin 23, a cytokine that contributes to inflammation in psoriasis.
- It is not approved to be used in children.
Humira was initially approved in 2002. In addition to plaque psoriasis, it is approved to treat other inflammatory conditions in adults such as rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis.
- Humira is classified as a tumor necrosis factor (TNF) blocker and works by reducing the inflammation linked with these conditions.
- Humira is also used in adults and children to treat Crohn's disease, juvenile idiopathic arthritis, ulcerative colitis, uveitis, and a skin condition called hidradenitis suppurativa.
Both Skyrizi (risankizumab-rzaa) and Humira (adalimumab) are biologics approved by the FDA to treat adults with moderate-to-severe plaque psoriasis. These medicines are used in patients who may benefit from taking injections, pills or phototherapy (treatment using ultraviolet light alone or with pills).
- Plaque psoriasis is a long-term autoimmune disease in which the cells of your skin are quickly replaced. This causes raised silvery plaques that can be flaky, red, and itchy.
- In autoimmune diseases the body's immune system starts to mistakenly attack healthy cells and tissues.
Use Skyrizi or Humira exactly as directed by your doctor.
Related Questions
- What does psoriasis look like?
- How do you get psoriasis and is it contagious?
- How does Tremfya work to treat plaque and psoriatic arthritis?
How do side effects compare between Skyrizi and Humira?
Skyrizi and Humira have some similar side effects and some that are different.
- Side effects that were reported commonly with Skyrizi (in >1% of patients with plaque psoriasis) include upper respiratory infections, headache, fatigue, injection site reactions and tinea (fungal) infections.
- The most common side effects with Humira (>10%) are infections (like upper respiratory infections or sinusitis), injection site reactions, headache and rash.
Learn more: Side effects for Skyrizi and Humira (in more detail)
How is dosing different between Skyrizi and Humira for psoriasis?
Both Skyrizi and Humira are given as subcutaneous (under the skin) injections for plaque psoriasis, but their dosing schedule is different.
- After 2 starter doses given 4 weeks apart, Skyrizi is given every 12 weeks (4 doses per year).
- One week after an initial starting dose, Humira is given as an injection every 2 weeks (26 doses per year).
Both Skyrizi and Humira come as single-dose, prefilled syringes or pens for injection for use in psoriasis. You or a caregiver can learn to give these injections at home, or your healthcare provider can administer these medicines. Do not attempt to give an injection until you (or a caregiver) have been taught how to do this correctly.
This is not all the information you need to know about Skyrizi or Humira for safe and effective use and does not take the place of talking to your doctor about your treatment. Review the full product information and discuss any questions you have with your doctor or other health care provider.
References
- Reich K, Gooderham, Thaci D et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): A randomized, double-blind, active-comparator-controlled phase 3 trial. Lancet 2019. Aug 17;394(10198):576-586. Erratum in: Lancet. 2019 Jul 16;: PMID: 31280967. doi: 10.1016/S0140-6736(19)30952-3
- Skyrizi prescribing information. 1/2022. AbbVie. North Chicago, Ill. Accessed Jan. 30, 2022 at https://www.rxabbvie.com/pdf/skyrizi_pi.pdf
- Humira prescribing information. 2/2021. AbbVie Inc. North Chicago, Ill. Accessed Sept. 13, 2021 at https://www.rxabbvie.com/pdf/humira.pdf
- Skyrizi.com. AbbVie. Skyrizi vs. Humira. Accessed Sept. 13, 2021 at https://www.skyrizihcp.com/clinical-efficacy/switch/humira-to-skyrizi
- Skyrizi.com. AbbVie. Accessed June 21, 2022 at https://www.skyrizi.com/psoriasis-treatment/switch-to-skyrizi
Read next
Related medical questions
- What are the new drugs for plaque psoriasis?
- Why should I take folic acid with methotrexate?
- Is triamcinolone acetonide an antifungal cream?
- How do you use clobetasol propionate on your scalp?
- Clobetasol vs. triamcinolone - how do they compare?
- What is a substitute for fluocinonide cream?
- Halobetasol vs. clobetasol - How do they compare?
- How long does methotrexate stay in your system?
- Does taking vitamin D help with psoriasis?
- What causes Plaque Psoriasis?
- Sotyktu vs Otezla: How do they compare?
- Can clobetasol be used for toenail fungus?
- How does Taltz compare to Cosentyx for psoriatic arthritis?
- How long does it take for Skyrizi to work?
- Is fluocinonide an antifungal cream?
- How long should you use fluocinonide for?
- How do you inject Humira?
- Does Feverfew interact with any drugs?
- How long does clobetasol stay in your system?
- Cosentyx vs Humira: How do they compare?
- Who is the actress in the Stelara commercial?
- What's the dosing schedule for Skyrizi?
- How long does it take for Sotyktu to work?
- What happens if Cosentyx is not refrigerated?
- Can Enstilar be used on the scalp?
- What is the mechanism of action of infliximab?
- What is fluocinonide cream good for?
- Does Cosentyx cause weight gain or loss?
- Does Skyrizi cause cancer?
- How long does it take for Tremfya to work?
Drug information
Related support groups
- Psoriasis (97 questions, 305 members)
- Humira (69 questions, 369 members)
- Skyrizi (16 questions, 6 members)
- Adalimumab (14 questions, 14 members)
- Risankizumab (8 questions, 3 members)
- Psoriatic Arthritis (78 questions, 276 members)
- Crohn's Disease (49 questions, 284 members)
- Plaque Psoriasis (81 questions, 69 members)
