Olumiant Dosage
Generic name: baricitinib 2mg
Dosage form: tablet, film coated
Drug classes: Antirheumatics, Selective immunosuppressants
Medically reviewed by Drugs.com. Last updated on Mar 20, 2023.
Recommended Evaluations and Immunization Prior to Treatment Initiation
Prior to OLUMIANT treatment initiation, consider performing the following evaluations:
- Active and latent tuberculosis (TB) infection evaluation – OLUMIANT should not be given to patients with active tuberculosis (TB). If latent infection is positive in patients with rheumatoid arthritis or alopecia areata, consider treatment for TB prior to OLUMIANT use [see Warnings and Precautions (5.1)].
- Viral hepatitis screening in accordance with clinical guidelines [see Warnings and Precautions (5.1)].
- Complete blood count – Assess baseline values and verify whether treatment can be initiated:
- -
- In patients with rheumatoid arthritis or alopecia areata, OLUMIANT initiation is not recommended in patients with an absolute lymphocyte count (ALC) <500 cells/μl, absolute neutrophil count (ANC) <1000 cells/μl, or hemoglobin level <8 g/dL.
- -
- In patients with COVID-19, OLUMIANT initiation is not recommended if the ALC is <200 cells/μl or if the ANC is <500 cells/μl.
Monitor complete blood counts during treatment and modify dosage as recommended [see Dosage and Administration (2.5) and Warnings and Precautions (5.7)].
- Baseline hepatic and renal function – Assess baseline values and monitor patients for laboratory changes. Modify dosage based on hepatic and renal impairment, and laboratory abnormalities [see Dosage and Administration (2.5) and Warnings and Precautions (5.7)].
In patients with rheumatoid arthritis or alopecia areata, update immunizations in agreement with current immunization guidelines [see Warnings and Precautions (5.9)].
Dosage Recommendations in Rheumatoid Arthritis
The recommended dosage of OLUMIANT is 2 mg once daily orally, with or without food [see Clinical Pharmacology (12.3)]. An alternative administration for patients unable to swallow tablets may be used [see Dosage and Administration (2.8)]. OLUMIANT may be used as monotherapy or in combination with methotrexate or other non-biologic DMARDs.
Dosage Recommendations in COVID-19
The recommended dosage of OLUMIANT for adults is 4 mg once daily orally, with or without food, for 14 days or until hospital discharge, whichever occurs first. An alternative administration for patients unable to swallow tablets may be used [see Dosage and Administration (2.8)].
Dosage Recommendations in Alopecia Areata
The recommended dosage of OLUMIANT is 2 mg once daily orally, with or without food. Increase to 4 mg once daily if the response to treatment is not adequate.
For patients with nearly complete or complete scalp hair loss, with or without substantial eyelash or eyebrow hair loss, consider treating with 4 mg once daily, with or without food.
Once patients achieve an adequate response to treatment with 4 mg, decrease the dosage to 2 mg once daily.
Dosage Modifications Due to Infections, Cytopenias and Anemia
Rheumatoid Arthritis and Alopecia Areata
- Avoid use of OLUMIANT in patients with active, serious or opportunistic infection, including localized infections. If a patient develops a serious infection hold treatment with OLUMIANT until the infection is controlled [see Warnings and Precautions (5.1)].
- Dosage modifications for patients with rheumatoid arthritis or alopecia areata and cytopenias or anemia are described in Table 1.
| Laboratory Analyte | Laboratory Analyte Value | Recommendation |
| Absolute Lymphocyte Count (ALC) | ≥500 cells/μL | Maintain dosage |
| <500 cells/μL | Interrupt OLUMIANT until ALC ≥500 cells/μL | |
| Absolute Neutrophil Count (ANC) | ≥1000 cells/μL | Maintain dosage |
| <1000 cells/μL | Interrupt OLUMIANT until ANC ≥1000 cells/μL | |
| Hemoglobin | ≥8 g/dL | Maintain dosage |
| <8 g/dL | Interrupt OLUMIANT until hemoglobin ≥8 g/dL |
COVID-19
- Monitor patients for signs and symptoms of new infections during treatment with OLUMIANT. The risks and benefits of treatment with OLUMIANT in COVID-19 patients with other concurrent infections should be considered [see Warnings and Precautions (5.1)].
- Dosage modifications for patients with COVID-19 and cytopenias are described in Table 2.
| Laboratory Analyte | Laboratory Analyte Value | Recommendation |
| Absolute Lymphocyte Count (ALC) | ≥200 cells/μL | Maintain dosage |
| <200 cells/μL | Interrupt OLUMIANT until ALC ≥200 cells/μL | |
| Absolute Neutrophil Count (ANC) | ≥500 cells/μL | Maintain dosage |
| <500 cells/μL | Interrupt OLUMIANT until ANC ≥500 cells/μL |
Dosage Modifications for Patients with Renal Impairment or Hepatic Impairment
Renal Impairment
Dosage modifications for patients with rheumatoid arthritis and renal impairment are described in Table 3.
| Renal Impairment Stage | Estimated Glomerular Filtration Rate (eGFR) | Recommendation |
| Mild | 60 – <90 mL/minute/1.73 m2 | 2 mg once daily |
| Moderate | 30 - <60 mL/min/1.73 m2 | 1 mg once daily |
| Severe | <30 mL/minute/1.73 m2 | Not recommended |
Hepatic Impairment
- OLUMIANT is not recommended for use in patients with severe hepatic impairment.
- Interrupt OLUMIANT, if increases in ALT or AST are observed and drug-induced liver injury (DILI) is suspected, until the diagnosis of DILI is excluded [see Warnings and Precautions (5.8)].
Renal Impairment
- Dosage modifications for patients with COVID-19 and renal impairment are described in Table 4.
| Renal Impairment Stage | Estimated Glomerular Filtration Rate (eGFR) | Recommendation |
| Mild | 60 - <90 mL/min/1.73m2 | 4 mg once daily |
| Moderate | 30 - <60 mL/min/1.73m2 | 2 mg once daily |
| Severe | 15 - <30 mL/min/1.73m2 | 1 mg once daily |
| End Stage Renal Disease, Patients on Dialysis, or Acute Kidney Injury | <15 mL/min/1.73m2 | Not recommended |
Hepatic Impairment
- It is not known if dosage adjustment is needed in patients with COVID-19 and severe hepatic impairment. OLUMIANT should only be used in patients with COVID-19 and severe hepatic impairment if the potential benefit outweighs the potential risk.
- Interrupt OLUMIANT, if increases in ALT or AST are observed and DILI is suspected, until the diagnosis of DILI is excluded [see Warnings and Precautions (5.8)].
Renal Impairment
Dosage modifications for patients with alopecia areata and renal impairment are described in Table 5.
| Renal Impairment Stage | Estimated Glomerular Filtration Rate (eGFR) | Recommendation | |
| If the recommended dosage is 2 mg once daily |
If the recommended dosage is 4 mg once daily |
||
| Mild | 60 – <90 mL/minute/1.73 m2 | Maintain dosage | |
| Moderate | 30 – <60 mL/min/1.73 m2 | Reduce to 1 mg once daily | Reduce to 2 mg once daily |
| Severe | <30 mL/minute/1.73 m2 | Not recommended | |
Hepatic Impairment
- OLUMIANT is not recommended for use in patients with severe hepatic impairment.
- Interrupt OLUMIANT, if increases in ALT or AST are observed and DILI is suspected, until the diagnosis of DILI is excluded [see Warnings and Precautions (5.8)].
Dosage Modifications Due to Drug Interactions
Rheumatoid Arthritis, COVID-19 or Alopecia Areata
The recommended dosage of OLUMIANT in patients taking strong Organic Anion Transporter 3 (OAT3) inhibitors, such as probenecid, are shown in Table 6 [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
| Concomitant Medication | Recommendation |
Strong OAT3 inhibitors (e.g., probenecid) |
If the recommended dosage is 4 mg once daily, reduce dosage to 2 mg once daily. |
| If the recommended dosage is 2 mg once daily, reduce dosage to 1 mg once daily. | |
| If the recommended dosage is 1 mg once daily, consider discontinuing probenecid. |
Alternative Administration for Patients Unable to Swallow Tablets
For patients who are unable to swallow whole tablets, an alternative mode of administration may be considered:
- Oral dispersion
- Gastrostomy tube (G tube)
- Nasogastric tube (NG tube) or orogastric tube (OG tube)
Intact tablets are not hazardous. Tablets may be crushed to facilitate dispersion. It is not known if powder from the crushed tablets may constitute a reproductive hazard to the preparer. If tablets are crushed, use proper control measures (e.g., ventilated enclosure) or personal protective equipment (i.e., N95 respirator). Dispersed tablets are stable in water for up to 4 hours.
Preparation Instructions for Alternative Administration:
- Oral administration of dispersed tablets in water: For patients who are unable to swallow whole tablets, 1-mg, 2-mg, or 4-mg baricitinib tablet(s), or any combination of tablets necessary to achieve the desired dose up to 4-mg may be placed in a container with approximately 10 mL (5 mL minimum) of room temperature water, dispersed by gently swirling the tablet(s) and immediately taken orally. The container should be rinsed with an additional 10 mL (5 mL minimum) of room temperature water and the entire contents swallowed by the patient (Table 7).
- Administration via G tube: For patients with a G tube, 1-mg, 2-mg, or 4-mg baricitinib tablet(s), or any combination of tablets necessary to achieve the desired dose up to 4-mg may be placed in a container with approximately 15 mL (10 mL minimum) of room temperature water and dispersed with gentle swirling. Ensure the tablet(s) are sufficiently dispersed to allow free passage through the tip of the syringe. Withdraw entire contents from the container into an appropriate syringe and immediately administer through the gastric feeding tube. Rinse container with approximately 15 mL (10 mL minimum) of room temperature water, withdraw the contents into the syringe, and administer through the tube (Table 7).
- Administration via NG or OG tube: For patients with a NG or OG tube, 1-mg, 2-mg, or 4-mg baricitinib tablet(s), or a combination of tablets necessary to achieve the desired dose up to 4-mg may be placed into a container with approximately 30 mL of room temperature water and dispersed with gentle swirling. Ensure the tablet(s) are sufficiently dispersed to allow free passage through the tip of the syringe. Withdraw the entire contents from the container into an appropriate syringe and immediately administer through the enteral feeding tube. To avoid clogging of small diameter tubes (smaller than 12 Fr), the syringe can be held horizontally and shaken during administration. Rinse container with a sufficient amount (minimum of 15 mL) of room temperature water, withdraw the contents into the syringe, and administer through the tube (Table 7).
| Administration via | Dispersion Volume | Container Rinse Volume |
| Oral dispersion | 10 mL | 10 mL |
| G tube | 15 mL | 15 mL |
| NG tube or OG tube |
30 mL | 15 mL |
DOSAGE FORMS AND STRENGTHS
OLUMIANT is available as debossed, film-coated tablets:
- 1 mg tablet contains a recessed area on each face of the tablet surface, is very light pink, round, debossed with “Lilly” on one side and “1” on the other.
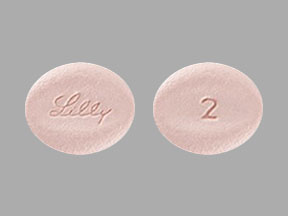
- 2 mg tablet contains a recessed area on each face of the tablet surface, is light pink, oblong, debossed with “Lilly” on one side and “2” on the other.
- 4 mg tablet contains a recessed area on each face of the tablet surface, is medium pink, round, debossed with “Lilly” on one side and “4” on the other.
Frequently asked questions
- What are the new drugs for rheumatoid arthritis (RA)?
- What are JAK inhibitors and how do they work?
- Which JAK inhibitors are approved in the U.S?
- How effective is Olumiant for alopecia (hair loss)?
- Is baricitinib (Olumiant) being used to treat COVID-19?
- What type of drug is Olumiant (baricitinib)?
More about Olumiant (baricitinib)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (11)
- Drug images
- Latest FDA alerts (1)
- Side effects
- During pregnancy
- FDA approval history
- Drug class: antirheumatics
- Breastfeeding
- En español
Patient resources
Professional resources
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.