Macugen Dosage
Generic name: PEGAPTANIB SODIUM 3.47mg in 1mL
Dosage form: injection, solution
Drug class: Anti-angiogenic ophthalmic agents
Medically reviewed by Drugs.com. Last updated on Jun 21, 2024.
Dosing
MACUGEN 0.3 mg should be administered once every six weeks by intravitreous injection into the eye to be treated.
Preparation for Administration
MACUGEN should be inspected visually for particulate matter and discoloration prior to administration. If visible particulates are observed and/or the liquid in the syringe is discolored, the syringe must not be used.
Administration of the syringe contents involves assembly of the syringe with the administration needle. The injection procedure should be carried out under controlled aseptic conditions, which includes the use of sterile gloves, a sterile drape, and a sterile eyelid speculum (or equivalent). When ready to assemble syringe and administer injection, carefully peel open pouches, remove contents, and place on sterile field. If upon opening the pouch, the plastic clip is missing or not attached to the syringe, the syringe should not be used.
To avoid compromising the sterility of the product, do not pull back on the plunger.
- 1.
- Remove the syringe from the plastic clip.
- 2.
- Twist off cap.
- 3.
- Attach the sterile, single-use administration needle (included) to the syringe by screwing it into the syringe tip.
--Another sterile, single-use administration needle may be used in lieu of the one included. Remove the plastic needle shield from the needle. - 4.
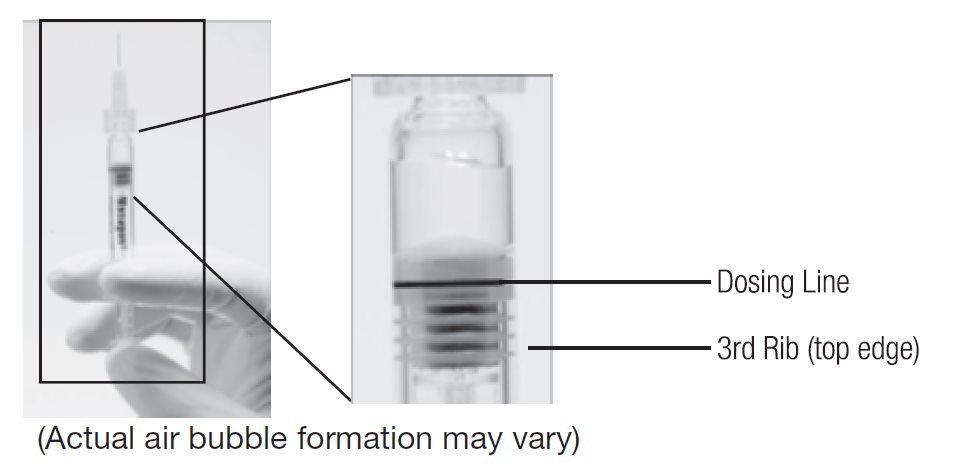
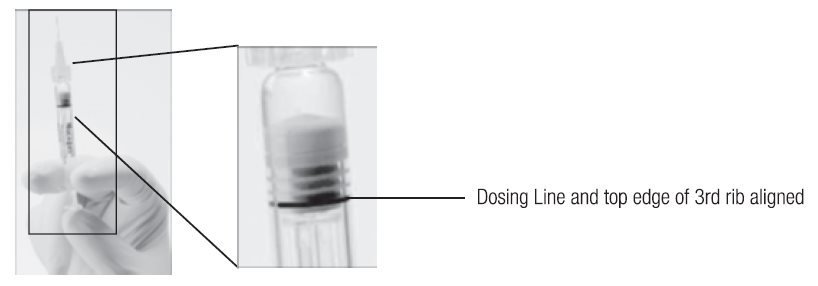
- Holding the syringe with the needle pointing up, check the syringe for bubbles. If there are bubbles, gently tap the syringe with your finger until the bubbles rise to the top of the syringe. SLOWLY depress the plunger to eliminate all the bubbles and to expel the excess drug so that the top edge of the 3rd rib on the plunger stopper aligns with the preprinted black dosing line (see Figure 2, below).
- 5.
- Inject the entire contents of the syringe.
PRIOR to Injection
Figure 1. Before expelling air bubble and excess drug
READY for Injection
Figure 2. After expelling air bubble and excess drug
Administration
The injection procedure should be carried out under controlled aseptic conditions, which includes the use of sterile gloves, a sterile drape, and a sterile eyelid speculum (or equivalent). Adequate anesthesia and a broad-spectrum microbicide should be given prior to the injection.
The patient's medical history for hypersensitivity reactions should be evaluated prior to performing the intravitreal procedure [see Warnings and Precautions (5) and Adverse Events (6)].
Following the injection, patients should be monitored for elevation in intraocular pressure and for endophthalmitis. Monitoring may consist of a check for perfusion of the optic nerve head immediately after the injection, tonometry within 30 minutes following the injection, and monitoring during the week following the injection. Patients should be instructed to report any symptoms suggestive of endophthalmitis without delay.
No special dosage modification is required for any of the populations that have been studied (i.e. gender, elderly).
The safety and efficacy of MACUGEN therapy administered to both eyes concurrently have not been studied.
More about Macugen (pegaptanib ophthalmic)
- Check interactions
- Compare alternatives
- Reviews (1)
- Side effects
- During pregnancy
- FDA approval history
- Drug class: anti-angiogenic ophthalmic agents
- Breastfeeding
Patient resources
Professional resources
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.