Casgevy Dosage
Generic name: exagamglogene autotemcel 130000001 in 1mL
Dosage form: injection, suspension
Drug class: Miscellaneous uncategorized agents
Medically reviewed by Drugs.com. Last updated on Jan 22, 2024.
For autologous use only. For one-time, single dose intravenous use only.
Dose
The minimum recommended dose of CASGEVY is 3 × 106 CD34+ cells/kg.
CASGEVY is provided as a single dose for infusion containing a suspension of CD34+ cells in one or more vials. See the Lot Information Sheet provided with the product shipment for additional information pertaining to the number of vials required to achieve the patient-specific dose. Administer all vials.
Preparation Before CASGEVY Infusion
Confirm that hematopoietic stem cell (HSC) transplantation is appropriate for the patient before mobilization, apheresis and myeloablative conditioning are initiated.
Screen patients for HIV-1, HIV-2, HBV, HCV, and any other infectious agents in accordance with local guidelines before collection of cells for manufacturing. CASGEVY should not be used in patients with active HIV-1, HIV-2, HBV or HCV.
Discontinue disease modifying therapies (e.g., hydroxyurea, crizanlizumab, voxelotor) 8 weeks before the planned start of mobilization and conditioning [see Drug Interactions (7.2, 7.3)].
Sickle Cell Disease
Prior to apheresis it is recommended that patients be transfused with a goal to maintain hemoglobin S (HbS) levels < 30% of total hemoglobin (Hb) while keeping total Hb concentration ≤ 11 g/dL.
Transfusion-dependent β-thalassemia
Prior to apheresis procedure it is recommended that patients be transfused with a goal to maintain hemoglobin (Hb) ≥ 11 g/dL.
Mobilization and Apheresis
Patients are required to undergo CD34+ HSC mobilization followed by apheresis to isolate the CD34+ cells needed for CASGEVY manufacturing.
Plerixafor and Granulocyte-Colony Stimulating Factor (G-CSF) were used for mobilization in patients with TDT.
Single agent plerixafor was used for mobilization in patients with SCD. G-CSF should not be administered for mobilization in patients with SCD.
Refer to the prescribing information for the mobilization agent(s) prior to treatment. See Clinical Studies (14) for description of the mobilization regimen used in the clinical trials.
Maximize CD34+ cell collection to obtain as many CD34+ cells as possible for product manufacturing during each mobilization and apheresis cycle. Perform two consecutive days of cell collection for product manufacturing per cycle, if clinically tolerated. A total collection target of at least 20 × 106 CD34+ cells/kg is recommended for product manufacture. Collected cells should be sent for product manufacturing even if the total collection target is not achieved. In addition, at least 2 × 106 CD34+ cells/kg is required to be collected for back-up unmodified rescue cells. A third day of cell collection can be used to obtain back-up rescue cells, if needed. If the minimum dose of CASGEVY (3 × 106 CD34+ cells/kg) is not met after initial product manufacturing, the patient will need to undergo additional cycles of mobilization and apheresis. Each mobilization and apheresis cycle must be separated by a minimum of 14 days.
The back-up collection of ≥ 2 × 106 CD34+ cells/kg of unmodified rescue cells must be collected from the patient and be cryopreserved prior to myeloablative conditioning and infusion with CASGEVY. The unmodified back-up cells may be needed for rescue treatment under any one of the following conditions: (1) compromise of CASGEVY after initiation of myeloablative conditioning and before CASGEVY infusion; (2) neutrophil engraftment failure; or (3) loss of engraftment after infusion with CASGEVY [see Warnings and Precautions (5.1)].
Myeloablative Conditioning
In patients with SCD it is recommended that patients be transfused at least 8 weeks prior to the initiation of myeloablative conditioning, with a goal of maintaining hemoglobin S (HbS) levels of < 30% of total Hb while keeping total Hb concentration ≤ 11 g/dL. At initiation of red blood cell exchange or simple transfusions, discontinue disease modifying therapies for sickle cell disease (e.g., hydroxyurea, crizanlizumab, voxelotor).
In patients with TDT it is recommended that patients be transfused to maintain hemoglobin (Hb) ≥ 11 g/dL for 60 days prior to myeloablative conditioning.
Stop iron chelation therapy at least 7 days prior to myeloablative conditioning.
Do not begin myeloablative conditioning until the complete set of vial(s) comprising the total dose of CASGEVY has been received and stored at the treatment center and the availability of the back-up collection of unmodified rescue cells is confirmed. See the Lot Information Sheet provided with the product shipment for confirmation of the total dose of CASGEVY.
Administer full myeloablative conditioning prior to treatment with CASGEVY 1. Refer to the prescribing information for the myeloablative conditioning agent prior to treatment. See Clinical Studies (14) for the conditioning regimen used in the clinical trials.
Consider administration of anti-seizure prophylaxis. Use agents other than phenytoin prior to initiating busulfan conditioning. Consider prophylaxis for hepatic veno-occlusive disease (VOD)/hepatic sinusoidal obstruction syndrome prior to initiating busulfan conditioning.
CASGEVY must be administered between 48 hours and 7 days after the last dose of the myeloablative conditioning.
Receipt and Storage of CASGEVY
- CASGEVY is shipped to the treatment center frozen in a vapor phase of liquid nitrogen shipper.
- Confirm patient identifiers on the product label(s) and Lot Information Sheet.
- If there are any concerns about the product or packaging upon receipt, contact Vertex at +1-877-634-8789.
- Transfer CASGEVY from the vapor phase of nitrogen shipper to the treatment center vapor phase of liquid nitrogen storage at ≤ -135 °C (≤ -211 °F).
Preparing for CASGEVY Administration
CASGEVY contains human cells. Follow universal precautions (wearing gloves, protective clothing, and eye protection) and local biosafety guidelines applicable for handling and disposal of such products to avoid potential transmission of infectious diseases. All material that has been in contact with CASGEVY (solid and liquid waste) should be handled and disposed of as potentially infectious waste in accordance with local biosafety guidelines.
Coordinate the timing of CASGEVY thaw and infusion. Confirm the infusion time in advance and adjust the start time for thaw so that CASGEVY is available for infusion when the patient and healthcare providers are ready. Thaw and infuse one vial at a time.
Premedication: Administer an antipyretic (e.g., acetaminophen) and an antihistamine (e.g., diphenhydramine hydrochloride) prior to administering CASGEVY.
Before thaw, confirm CASGEVY is printed on the vial label and the patient's identity matches the unique patient information located on the CASGEVY vial(s). Do not infuse CASGEVY if the information on the patient-specific label on any of the vials does not match the intended patient, and contact Vertex at +1-877-634-8789.
A dose of CASGEVY may be contained in one or more cryopreserved patient-specific vial(s). Ensure that the correct number of vials are present. Use the accompanying Lot Information Sheet to confirm the total number of vials to be administered and confirm that each vial is within the expiration date prior to preparation of CASGEVY for infusion.
Inspect the vial(s) for any breaks or cracks prior to thawing. If a vial is compromised, do not infuse the contents. Call Vertex at +1-877-634-8789.
When the dose consists of multiple vials, thaw and administer one vial at a time. While thawing and administering a vial, remaining vials must remain in cryostorage at ≤ -135 °C (≤ -211 °F).
Assemble supplies needed to thaw and withdraw the product from the vial(s). With the exception of the water bath, these supplies are single use. Assemble sufficient supplies for each vial to be administered:
- Water bath
- Alcohol swabs
- Vial adapter (to allow for needleless extraction)
- 18-micron stainless steel filter
- 30 mL luer-lock syringe
- 0.9% sodium chloride (saline, 5 to 10 mL needed for each vial)
- 10 mL luer-lock syringe for saline rinse
Thawing the CASGEVY vials
- Thaw each CASGEVY vial at 37 °C (98 °F) using a water bath. Thaw each vial holding the vial neck, gently agitating clockwise and counterclockwise.
- Thawing of each vial can take between 10 to 15 minutes. Thawing is complete when ice crystals are no longer visible in the vial. Ensure water bath temperature does not exceed 40 °C (104 °F) during thawing.
- Do not leave CASGEVY unattended during thaw.
- Remove vial from water bath immediately once thawed.
- The thawed product should appear as a cellular suspension, which may contain proteinaceous particles or cellular aggregates.
- Inspect the vial(s) for any breaks or cracks after thawing.
- Do not wash, spin down and/or resuspend CASGEVY in new media prior to infusion.
- Do not sample, alter, irradiate, or refreeze CASGEVY.
- Infuse within 20 minutes of thaw.
Administration
CASGEVY is for autologous use only. Before infusion, confirm that the patient's identity matches the unique patient identifiers on the CASGEVY vial(s). Do not infuse CASGEVY if the information on the patient-specific label does not match the intended patient.
A patient's dose may consist of multiple vials. All vials must be administered. Use the Lot Information Sheet to confirm the total number of vials to be administered. The entire volume of each vial provided should be infused. If more than one vial is provided, administer each vial completely before proceeding to thaw and infuse the next vial.
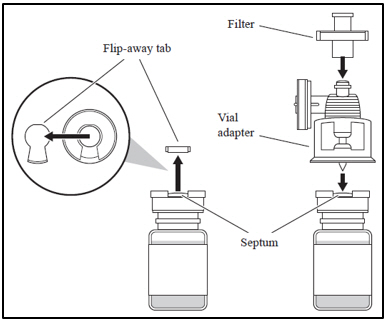
| 1. Attaching the vial adapter and filter | |
|
|
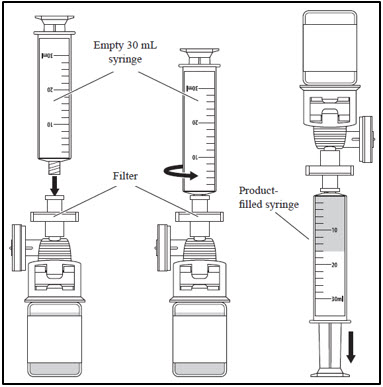
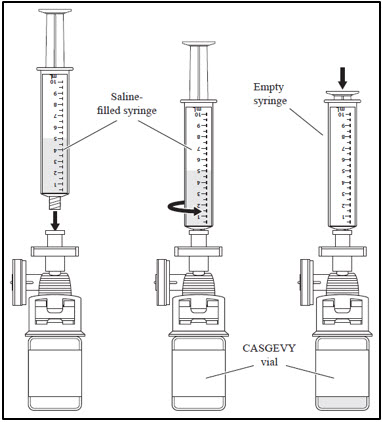
| 2. Withdrawing CASGEVY from the vial | |
|
|
|
|
|
|
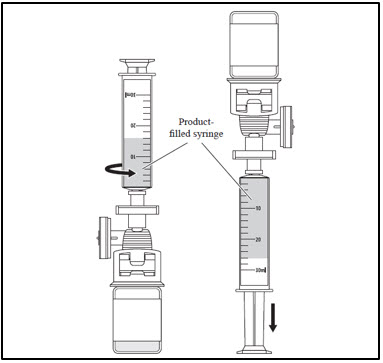
| 3. Administer CASGEVY through central venous catheter | |
|
|
Repeat steps 1-3 for each remaining vial. If more than one vial is needed to achieve the patient-specific dose, administer each vial completely before proceeding to thaw and infuse the next vial.
After CASGEVY Administration
Follow standard procedures for patient management after HSC transplantation after CASGEVY infusion.
- Irradiate any blood products required within the first 3 months after CASGEVY infusion.
- Patients treated with CASGEVY should not donate blood, organs, tissues, or cells at any time in the future.
- Restarting iron chelation after CASGEVY infusion may be necessary and should be based on clinical practice. Avoid the use of non-myelosuppressive iron chelators for at least 3 months and use of myelosuppressive iron chelators for at least 6 months after CASGEVY infusion. Phlebotomy can be used in lieu of iron chelation, when appropriate [see Drug Interactions (7.4)].
More about Casgevy (exagamglogene autotemcel)
- Check interactions
- Compare alternatives
- Side effects
- During pregnancy
- FDA approval history
- Drug class: miscellaneous uncategorized agents
- En español
Patient resources
Professional resources
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.