Anesketin (Canada)
This treatment applies to the following species: Company: Dechra
Company: Dechra
Ketamine hydrochloride injection, Mfr. Std.
Veterinary Use Only
DIN 02534576
Sterile
Description
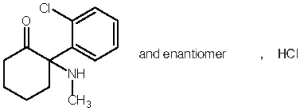
Anesketin (ketamine hydrochloride) is a rapid acting, nonbarbiturate general anesthetic for cats, chemically designated 2-(o-chlorophenyl)-2-methylamino-cyclohexanone hydrochloride. It is supplied as a slightly acid (pH 3.5 - 5.0) solution for intramuscular injection in a concentration containing 115.4 mg/mL ketamine hydrochloride, equivalent to 100 mg/mL ketamine base, and contains 1.0 mg/mL chlorocresol as a preservative, sodium hydroxide and/or hydrochloric acid for pH adjustment, and water for injection q.s. as non-medicinal ingredients.
Anesketin Indications
Anesketin (ketamine hydrochloride) is an anesthetic which may be used as a sole agent for restraint and for minor surgical procedures where muscle relaxation is not required in the domestic cat. Additional analgesia should be provided as needed.
Anesketin Dosage And Administration
Anesketin (ketamine hydrochloride) is administered by intramuscular injection.
Withholding food for at least 6 hours prior to induction of anesthesia by Anesketin is recommended when preparing for elective surgery.
Dosage:
11-33 mg/kg body weight depending on the degree of restraint or the type of minor surgical procedure that is intended. The following dosages are indicated as a guide but may need to be adjusted depending on the physical condition of the patient and the use of sedatives and premedication drugs.
|
Dose mg/kg |
Clinical Procedure |
|
11-22 |
Minor Restraint |
|
22-33 |
Minor surgery and restraint of fractious cats |
Contraindications
The use of Anesketin (ketamine hydrochloride) is not indicated in kittens under 12 weeks of age as it is not unusual in immature cats for anesthesia to be of shorter duration. Ketamine hydrochloride is contraindicated for procedures in cats requiring complete skeletal muscle relaxation.
Anesketin Cautions
For use in cats only. Because the drug is metabolized in the liver and excreted in the urine, although in cats largely unchanged, caution should be exercised in dosing subjects with hepatic or renal impairment. Caution should also be exercised in administering the drug to animals with cardiovascular abnormalities due to its hypertensive effect in cats. Safe use during pregnancy has not been established.
Induction and recovery should occur in quiet and calm surroundings.
Use of premedication drugs should be followed by a suitable reduction in ketamine dosage.
Eyes remain open and the pupils dilated; they should be protected by bland ophthalmic ointment or covering with a damp gauze swab.
WARNINGS
Keep out of reach of children.
Adverse Reactions
At high dosage, respiratory depression may occur. If at any time cyanosis occurs, or if respiration becomes excessively depressed, resuscitative measures should be instituted promptly, e.g., artificial respiration, oxygen administration, etc. May cause salivation in cats; atropine premedication may reduce this effect. Muscular twitching and mild tonic convulsions have occurred in the cat at recommended dose rates; these may subside spontaneously but may be prevented by acepromazine or controlled by use of acepromazine or ultra-short acting barbiturates in low doses. Use of intramuscular route may be associated with pain. Adverse reactions reported have included emesis, salivation, vocalization, erratic recovery and prolonged recovery, spastic jerking movements, convulsions, muscular tremors, hypertonicity, opisthotonos, dyspnea and cardiac arrest.
Clinical Pharmacology
Anesketin (ketamine hydrochloride) is a rapid acting anesthetic producing an anesthetic state characterized by profound analgesia, normal pharyngeal - laryngeal reflexes and skeletal muscle tone, mild cardiac stimulation and some respiratory depression. The anesthetic state produced by ketamine has been termed “dissociative anesthesia” in that it appears to selectively interrupt association of the brain before producing somesthetic sensory blockade.
Following administration of recommended doses of Anesketin (ketamine hydrochloride), blood pressure and heart rate are usually moderately and transiently increased. Respiratory rate, on the other hand, is usually decreased in cats. The pharyngeal reflexes are maintained thus aiding in the maintenance of a patent airway. Although some salivation is occasionally noted, the persistence of the swallowing reflex effectively reduces the hazards of salivation.
Other reflexes, e.g., corneal, pedal, etc., are maintained under Anesketin (ketamine hydrochloride) anesthesia, and should not be used as criteria for depth of anesthesia. Moreover, the eyes normally remain open with the pupil dilated, making it prudent to apply a bland ointment if anesthesia is to be prolonged. By single intramuscular injection in cats Anesketin (ketamine hydrochloride) has a wide margin of safety.
Following administration of recommended doses of Anesketin (ketamine hydrochloride) most cats become ataxic in about 5 minutes, and anesthesia will normally last 30-45 minutes. Recovery is generally smooth and uneventful, especially if animals are not stimulated by sound or handling during the recovery period. At the lower doses complete recovery usually occurs in 4-5 hours but with higher levels of Anesketin (ketamine hydrochloride) recovery time is more prolonged and less predictable. With high levels complete recovery may take 24 hours or more in some selected cases, especially if the patient is in poor condition or suffering from nephritis.
Storage
Store between 15 and 25°C in original packaging to protect from light. Contents should be used within 28 days after first use.
NOTE: Color of solution may vary from colorless to very slightly yellowish and may darken upon prolonged exposure to light. Do not use if particulate material appears.
Presentations
Multidose 50 mL vials.
Eurovet Animal Health B.V., Handelsweg 25, 5531 AE Bladel, The Netherlands
IMPORTED and DISTRIBUTED BY
Dechra Veterinary Products Inc., 1 Holiday Avenue, East Tower, Suite 345, Pointe-Claire, Quebec, H9R 5N3, Canada
Date of last revision: December 2022
618447
CPN: 1786081.0
1 HOLIDAY AVE., EAST TOWER SUITE 345, POINT-CLAIRE, QC, H9R 5N3
| Toll-Free: | 855-332-9334 | |
| Technical Services: | 855-332-9334 Option 1 | |
| Technical Services Email: | technical.ca@dechra.com | |
| Website: | www.dechra.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
