Zyvana Prescribing Information
Package insert / product label
Generic name: ascorbic acid, cholecalciferol, pyridoxine hydrochloride, zinc gluconate and selenium
Dosage form: capsule
Drug class: Vitamin and mineral combinations
Medically reviewed by Drugs.com. Last updated on Feb 22, 2024.
On This Page
Zyvana Description
Zyvana is an orally administered prescription dietary supplement formulation.
Zyvana should be administered under the supervision of a licensed medical practitioner.
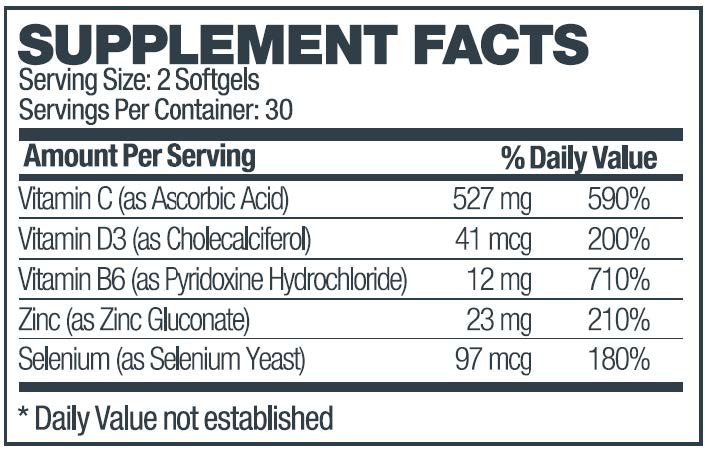
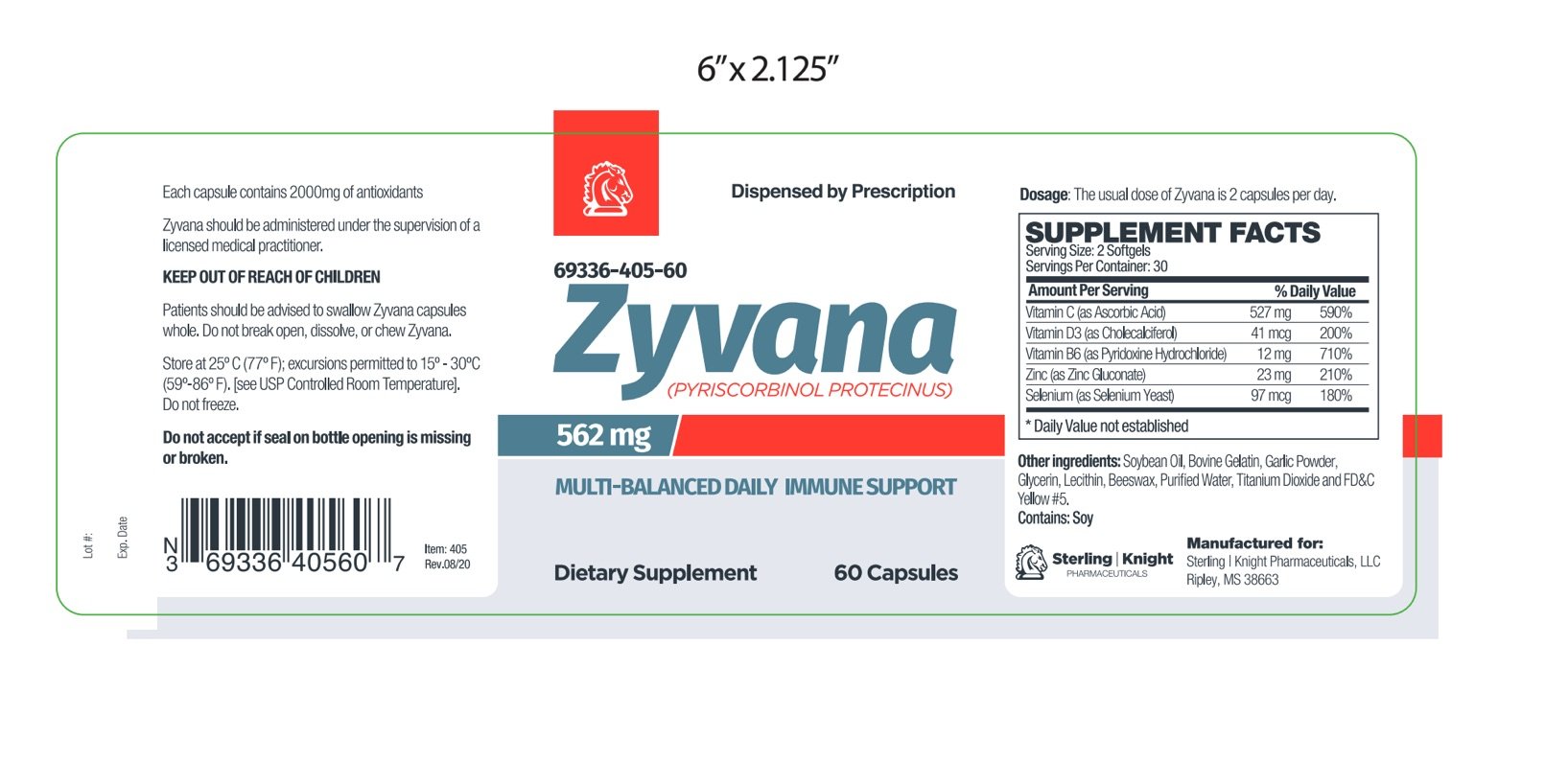
Other Ingredients: Soybean Oil, Gelatin (bovine), Garlic Powder, Glycerin, Lecithin, Beeswax, Purified Water, Titanium Dioxide, FD&C Yellow #5.
Indications and Usage for Zyvana
Zyvana is an orally administered prescription dietary supplement formulation for the management of suboptimal nutritional status in patients where advanced supplementation is required and nutritional supplementation in physiologically stressful conditions for maintenance of good health is needed.
Warnings and Precautions
This product is contraindicated in patients with a known hypersensitivity to any of the ingredients.Zyvana should only be used under the direction and supervision of a licensed medical practitioner. Use with caution in patients that may have a medical condition, are pregnant, lactating, trying to conceive, under the age of 18, or taking medications.
Zyvana Dosage and Administration
The usual dose of Zyvana is 2 capsules per day.The daily dose may be taken as a single dose of 2 capsules or 1 capsule twice daily.
How is Zyvana supplied
Zyvana is supplied as yellow softgel capsule printed with 405 dispensed in white HDPE plastic bottles of 60ct. 69336-405-60
Storage and Handling
Store at controlled room temperature 15°-30°C (59°F-86°F). Keep in cool dry place. Call your doctor about side effects. You may report side effects to FDA at 1-800-FDA-1088. KEEP THIS OUT OF THE REACH OF CHILDREN.
Reserved for Professional Recommendation
All prescriptions using this dietary supplement product shall be pursuant to state statutes as applicable. This is not an Orange Book product. This product may be administered under a physician’s supervision. There are no implied or explicit claims on therapeutic equivalence.
| ZYVANA
zyvana capsule |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Sterling-Knight Pharmaceuticals, LLC (079556942) |
More about multivitamin with minerals
- Check interactions
- Compare alternatives
- Reviews (185)
- Drug images
- Side effects
- Dosage information
- Drug class: vitamin and mineral combinations
- En español
Patient resources
Professional resources
Other brands
Dolomite, Centratex, Menatrol, Strovite One, ... +8 more