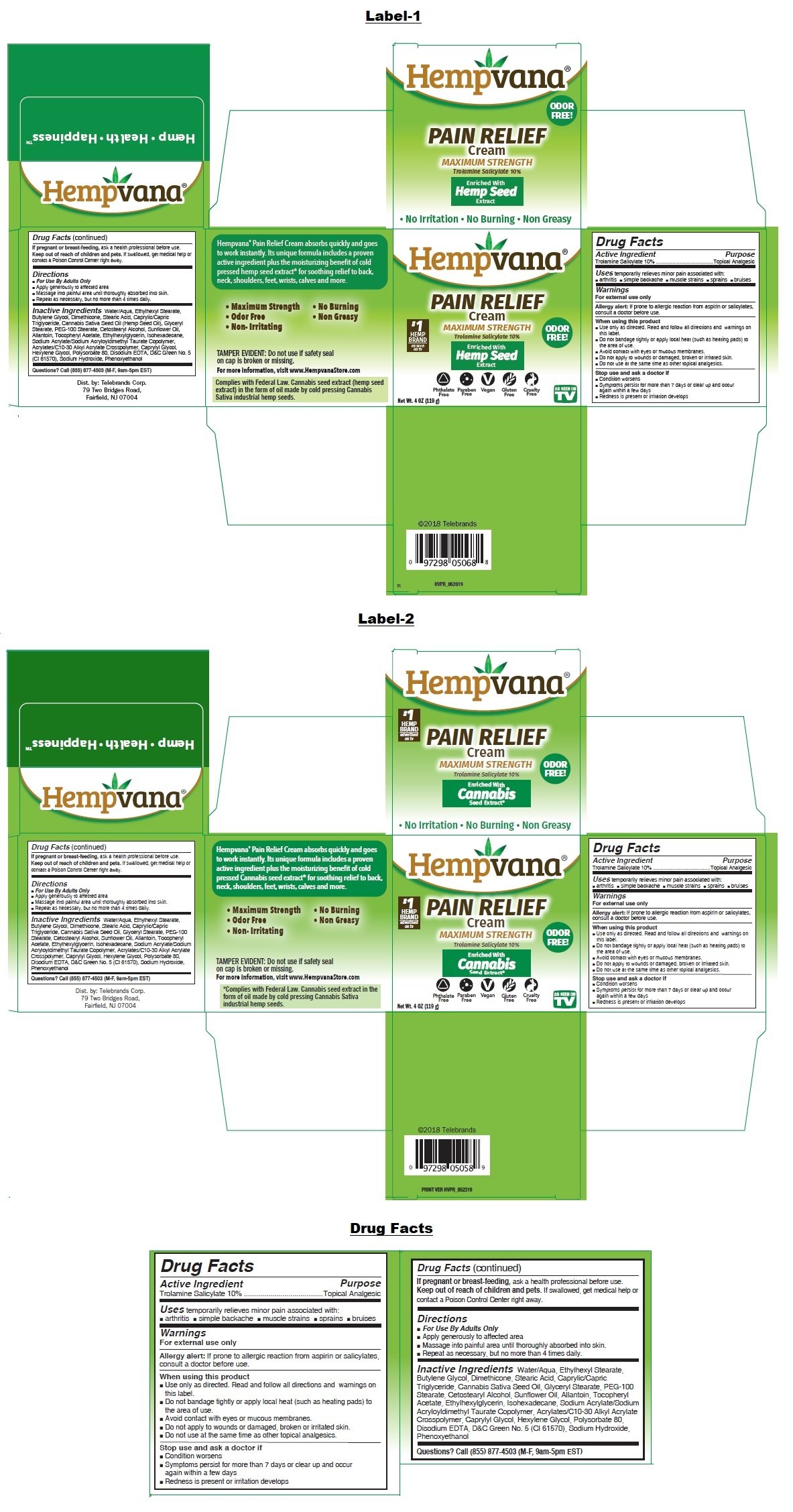
Hempvana Pain Relief
Dosage form: cream
Ingredients: TROLAMINE SALICYLATE 10g in 100g
Labeler: Neutraderm, Inc.
NDC code: 39765-011
Medically reviewed by Drugs.com. Last updated on Jun 8, 2023.
Trolamine Salicylate (10%)
Topical Analgesic
temporarily relieves minor pain associated with:
• arthritis • simple backache • muscle strains • sprains • bruises
For external use only
Allergy alert: If prone to allergic reaction from aspirin or salicylates, consult a doctor before use.
When using this product
- Use only as directed. Read and follow all directions and warnings on this label.
- Do not bandage tightly or apply local heat (such as heating pads) to the area of use.
- Avoid contact with eyes or mucous membranes.
- Do not apply to wounds or damaged, broken or irritated skin
- Do not use at the same time as other topical analgesics.
Stop use and ask a doctor if
- Condition worsens
- Symptoms persist for more than 7 days or clear up and occur again within a few days
- Redness is present or irritation develops
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children and pets. If swallowed, get medical help or contact a Poison Control Center right away.
- For Use By Adults Only
- Apply generously to affected area.
- Massage into painful area until thoroughly absorbed into skin.
- Repeat as necessary, but no more than 4 times daily.
Water/Aqua, Ethylhexyl Stearate, Butylene Glycol, Dimethicone, Stearic Acid, Caprylic/Capric Triglyceride, Cannabis Sativa Seed Oil (Hemp Seed Oil), Glyceryl Stearate, PEG-100 Stearate, Cetostearyl Alcohol, Sunflower Oil, Allantoin, Tocopheryl Acetate, Ethylhexylglycerin, Isohexadecane,
Sodium Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Caprylyl Glycol, Hexylene Glycol, Polysorbate 80, Disodium EDTA, D&C Green No. 5 (CI 61570), Sodium Hydroxide, Phenoxyethanol
Call (855) 877-4503 (M-F, 9am-5pm EST)
MAXIMUM STRENGTH
Enriched With Hemp Seed* Extract
ODOR FREE!
Phthalate Free Paraben Free Vegan Gluten Free Cruelty Free
• No Irritation • No Burning • Non Greasy
Hempvana® Pain Relief Cream absorbs quickly and goes to work instantly. Its unique formula includes a proven active ingredient plus the moisturizing benefit of cold pressed hemp seed* extract for soothing relief to back, neck, shoulders, feet, wrists, calves and more.
TAMPER EVIDENT: Do not use if safety seal on cap is broken or missing.
For more information, visit www.HempvanaStore.com
*Complies with Federal Law. Hemp seed extract contains no detectible tetrahydrocannabinol (THC) or cannabidiol (CBD), Derived from the cannabis industrial hemp cultivar.
Dist. by: Telebrands Corp.
79 Two Bridges Road,
Fairfield, NJ 07004
Hemp • Health • HappinessTM
| HEMPVANA
PAIN RELIEF
trolamine salicylate cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Neutraderm, Inc. (146224444) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Neutraderm, Inc. | 146224444 | manufacture(39765-011) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.