The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
Zeuterin Injectable Solution
This page contains information on Zeuterin Injectable Solution for veterinary use.The information provided typically includes the following:
- Zeuterin Injectable Solution Indications
- Warnings and cautions for Zeuterin Injectable Solution
- Direction and dosage information for Zeuterin Injectable Solution
Zeuterin Injectable Solution
This treatment applies to the following species: Manufacturer: Ark Sciences
Manufacturer: Ark Sciences
(Zinc Gluconate Neutralized by Arginine)
Chemical Sterilant
Zeuterin Injectable Solution Caution
Federal Law restricts this drug to use by or on the order of a licensed veterinarian.
Description
Sterile intratesticular injectable aqueous solution containing 0.2 M zinc gluconate neutralized to pH 7.0 with 0.2 M L-arginine (13.1 mg zinc per milliliter).
Active Ingredient
Each mL contains
|
Zinc as Zinc Gluconate |
13.1 mg |
Other Ingredients:
|
L-Arginine |
34.8 mg |
|
Water for Injection |
q.s. |
HCl to adjust to pH 7
Zeuterin Injectable Solution Indications
Zeuterin™ Injectable Solution is indicated for chemical sterilization in 3 to 10 month old male dogs.
Zeuterin Injectable Solution Dosage And Administration
Food should be withheld for 12 hours prior to injection to help prevent vomiting.
The drug is administered as one injection per testicle. Dose is based on testicular width (See Table 1) and is determined by measuring each testicle at its widest point using the caliper provided. Use a 1 mL syringe with a 28 gauge, 1/2 inch needle for injection of Zeuterin.
Table 1: Dose Corresponding to Testicular Width
|
Range of Testicular Width (mm) |
Dose Administered (mL) |
|
10-12 |
0.2 |
|
13-15 |
0.3 |
|
16-18 |
0.5 |
|
19-21 |
0.7 |
|
22-24 |
0.8 |
|
25-27 |
1.0 |
Testicular Measurement and Injection Procedure
Observe the proper testicular measurement and injection technique as demonstrated in the Zeuterin™ Injection Procedure video.
Testicular Measurement:
1. Check to see that the caliper is clean.
2. With the dog lying on its back, measure the width of each testicle by placing the testicle inside the two measuring points and then slide the ruler until it rests gently against the testicle.
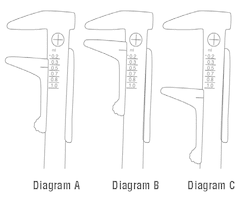
3. Read the dose corresponding to the measurement by noting the position of the indicator line on the sliding ruler. If the indicator line exceeds the dosage line, use the next higher dose (See Diagram A).
4. The testicle is too small for injection if the indicator line does not fall on or below the minimum position marked on the caliper (See Diagram B).
5. The testicle is too large for injection if the indicator line falls beyond the maximum position for the 1.0 mL dose marked on the caliper (See Diagram C).
Injection Procedure:
1. Chemical restraint should be considered to prevent the dog from moving during injection (See Warnings).
2. Use three needles and two syringes-one needle to withdraw the product into the syringes, one needle and one syringe for the dog’s right testicle and one needle and one syringe for the dog’s left testicle. Each testicle should be injected with a separate sterile needle. Use a 1 ml syringe with 28 gauge, 1/2 inch needle for injection of Zeuterin™. Larger gauge needles may cause drug to leak from the injection site (See Warnings).
3. Position the dog so that it is lying on its back for testicular measurement and intratesticular injection.
4. Use the caliper provided to measure the width of each testicle at the widest point and determine the dose for each testicle (See Testicular Measurement).
5. Withdraw into each syringe the correct dose of Zeuterin™ to be injected into each testicle according to testicular measurement.
6. Prepare the scrotum with an appropriate disinfectant. Avoid use of alcohol as it irritates the scrotal skin of some dogs.
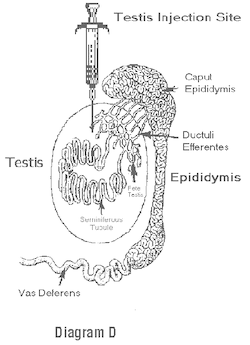
7. Hold the testicle firmly in one hand (do not squeeze) and use the other hand to hold the syringe filled with the dose for that testicle. Pull the skin tightly over each testicle to avoid injection into the scrotal sac or into the scrotal skin (See Warnings). Insert the needle into the dorsal cranial portion of the testicle beside the caput (head) epididymis (See Diagram D).
8. Inject slowly. Rapid injection may stimulate contraction of the seminiferous tubules and cause drug to leak from the injection site (See Warnings).
9. Do not use excessive injection pressure to force the drug into the testicle. If resistance is felt, discontinue the injection immediately. Do not re-inject. Zeuterin™ cannot be safely used in this dog.
10. Repeat the procedure for the remaining testicle. It is recommended as standard procedure to inject the right testicle first and then the left testicle to avoid confusion and re-injecting the same testicle.
Contraindications
Do not use Zeuterin™ in dogs with:
• Undescended testicles (cryptorchid).
• A disease or malformation of the testicle (including fibrosis of the testicles or epididymides).
• A history of allergic reaction to any of the components of the drug.
• Pre-existing scrotal irritation or dermatitis, transmissible venereal tumor (TVT).
Warnings
Human Warnings: Keep this and all drugs out of the reach of children. Not for human use. Wash the skin with soap and water and flush eyes with copious amounts of water if contact occurs. Flush mouth with water and drink plenty of water if accidental ingestion occurs. Contact a physician in cases of accidental exposure by any route (oral, dermal, or injection).
Animal Safety Warnings: Proper injection technique and post injection care are critical to the safe use of Zeuterin™.
Do not inject Zeuterin™ into the scrotal sac or scrotal skin. Contact between the drug and the scrotal skin activates collagenase enzymes, which may result in scrotal irritation, dermatitis, ulceration or necrosis. Chemical restraint should be used, if necessary, to prevent the dog from moving during the injection. To avoid leakage of drug from the injection site use only a 28 gauge 1/2 inch needle, inject slowly and immediately stop the injection if you feel resistance. Do not attempt to re-inject Zeuterin™ if you feel resistance to the injection. If you suspect that the drug was injected improperly into the scrotal sac or has contacted the scrotal skin, the dog should be closely monitored for up to 7 days post-injection for local adverse reactions.
Do not allow dogs to bite or lick the scrotum after injection. Monitor dogs closely while in the veterinary facility and for at least 7 days following release from the veterinary facility for signs of scrotal inflammation. Leash walk only and do not allow the scrotum to contact hard, wet surfaces as this may result in irritation, dermatitis, ulceration or necrosis. Distribute the Client Information Sheet to each client for proper care post-injection.
Do not inject Zeuterin™ more than once into each testicle.
PRECAUTIONS
1. To avoid irritation to the scrotal skin, do not shave or clip the scrotal hair. Use a nonalcoholic disinfectant as an aseptic agent.
2. Use this product only in healthy male dogs following a thorough examination of the scrotum to ensure the scrotum is free of skin irritation and ulceration and that both testicles are descended and normal as determined by digital palpation by the examining veterinarian.
3. The safety and effectiveness of Zeuterin has not been established in dogs less than 3 months of age or in dogs greater than 10 months of age.
4. Do not use if the testicular width is less than 10 mm or greater than 27 mm.
5. Obtain an accurate measurement of testicular widths by using the caliper provided and the dose corresponding to testicular measurement. Both testicles must be injected with the appropriate dose using the correct procedure in order to minimize adverse reactions and achieve sterility.
6. In dose determination and field studies, the most serious cases of scrotal irritation and ulceration occurred as a result of improper injection technique or were associated with the dog biting or licking the injection site after release to the owner. Detailed instructions on proper care post-injection should be provided to the owner via the attached Client Information Sheet (CIS).
Adverse Reactions
In a field study with 270 dogs, Zeuterin™ caused both local adverse reactions at the injection site and systemic reactions (See Table 2).
Zeuterin™ injection was observed to be painful in 2.6% of 270 treated dogs. Six dogs vocalized and one dog kicked following injection. Apparent scrotal pain post-injection was the most commonly reported local reaction (6.3%), most frequently seen during the first two days post-injection.
The most commonly reported systemic reactions to the Zeuterin™ injection were neutrophilia (6.3%), vomiting (4.4%), anorexia (4.1%) and lethargy (2.2%). These reactions were typically seen within 7 days of the injection. However, vomiting was most commonly seen on the day of the injection, between 1 minute and 4 hours post-injection. Six of 10 dogs that vomited did so more than once during this period. Withholding food for 12 hours prior to injection may prevent this from occurring.
The most severe reactions occurred when dogs bit or licked the scrotum following injection (See Warnings). These severe reactions were seen in < 1% of 270 dogs. One dog was returned to the clinic on Day 3 for an ulcerated scrotum. The wound healed with medical therapy. The second dog was reported with a perforated scrotum and a severe scrotal infection on Day 17 post-injection. The dog had licked and chewed through the scrotum down to the testicle. Surgical castration and scrotal ablation were performed.
Table 2: Adverse Reactions
|
Adverse Reactions |
No. of Animals (n = 270) |
Percent (%) |
|
Reaction Upon Injection |
||
|
Vocalization |
6 |
2.2% |
|
Kicking |
1 |
0.4% |
|
Local Reactions |
||
|
Scrotal Pain* |
17 |
6.3% |
|
Scrotal Irritation |
3 |
1.1% |
|
Biting and Licking |
2 |
0.7% |
|
Scrotal Swelling |
2 |
0.7% |
|
Scrotal Irritation and Dermatitis |
2 |
0.7% |
|
Scrotal Ulceration |
1 |
0.4% |
|
Scrotal Infection |
1 |
0.4% |
|
Dry Scrotal Skin |
1 |
0.4% |
|
Scrotal Bruising |
1 |
0.4% |
|
Preputial Swelling |
1 |
0.4% |
|
Scrotal Sore |
1 |
0.4% |
|
Systemic Reactions |
||
|
Neutrophilia |
17 |
6.3% |
|
Vomiting** |
12 |
4.4% |
|
Anorexia |
11 |
4.1% |
|
Lethargy |
6 |
2.2% |
|
Diarrhea |
5 |
1.9% |
|
Leukocytosis |
2 |
0.7% |
*Most scrotal pain was reported on the first two days after injection.
**Ten of the 12 dogs vomited within 1 minute and 4 hours after the injection.
To report suspected adverse events and/or obtain a copy of the MSDS or for technical assistance call Ark Sciences, Inc. at 1-800-345-4735. Adverse events can also be reported to the FDA by telephone at 1-888-FDA-VETS or online at http://www.fda.gov/AnimalVeterinary/SafetyHealth.
Information For Owner Or Person Treating Animals
Transient testicular swelling is an expected reaction to the injection. Field and dose determination data indicate that the swelling begins 24 hours post-injection and peaks at 48 hours post-injection. By 1 Month post-injection, most testicles will be atrophied. However, the degree of atrophy will vary individually and there may be variability between the left and right testicles of the same dog. This should be considered an expected response to the injection.
Zeuterin™ may not kill sperm present at the time of injection. Therefore, keep treated dogs away from females in heat for at least 60 days following the injection.
Unlike surgical castration, dogs treated with Zeuterin™ become sterile without removal of the testicles and, therefore, testosterone is not completely eliminated. Diseases which occur as a result of or in conjunction with testosterone hormones (prostatic disease, testicular or perianal tumors) may not be prevented.
As with surgical castration, secondary male characteristics (roaming, marking, aggression, or mounting) may be displayed.
Clinical Pharmacology
Zeuterin™ is a necrotizing agent that has a local effect when injected into the testicle. Based on histopatholology, one or more of the following events occur after injection of Zeuterin™.
• Atrophy of the testicles, epididymides, seminiferous tubules, and prostate gland.
• Scar tissue formation which prevents movement of sperm from the seminiferous tubules to the epididymis.
Effectiveness
The effectiveness of Zeuterin™ was evaluated in a field study of 270 male dogs of various breeds between 3-10 months of age. Of the 270 dogs that started the study, 224 completed the study to Month 6 and were included in the effectiveness evaluation. Semen analyses1 were conducted at 2, 6, and 12 Months post-injection. Dogs had to be aspermic2, azoospermic3, necrospermic4, or oligospermic5 at the Month 6 evaluation to be considered a treatment success. One injection of Zeuterin™ in each testicle produced successful sterilization in 223/224 dogs.
One treatment failure occurred in the field study. The Month 6 semen analysis for one dog revealed 100% motility and a sperm concentration of 165 million. Two dogs that were sterile at Month 6 and, therefore, deemed treatment successes, had sperm at month 12. One dog was azoospermic at Month 6 but at Month 12 was oligospermic (19 million sperm) with 100% motility. The second dog was oligospermic (10 million sperm) with 50% motility at Month 6, but at Month 12 had a sperm concentration of 49 million with 80% motility.
In a dose determination study, 30 male Beagle dogs, 6 Months of age, were injected with Zeuterin™ and followed for 2 years. Ten dogs were treated with a placebo. All dogs were exposed to untreated females in heat during the first 12 Months. Seven out of the 10 dogs in the control group mated with the females and 100% of these matings resulted in pregnancy. In the Zeuterin™-treated dogs, up to 40% mated with the females and 0% resulted in pregnancy. Two Zeuterin™-treated males, the only treated males with sperm in the ejaculate from Months 12-24, mounted the females but no pregnancies resulted. One female was artificially inseminated using one of the dog’s semen but a pregnancy did not result.
Mean serum testosterone levels were 41 to 52% lower in the groups treated with Zeuterin™ compared to the control group throughout the dose determination study. However, there were dogs in all treated groups that had testosterone levels similar to those for the control dogs at Months 1, 3, 6, and 9 and from 12 to 24 months post-injection. By Month 24, the testosterone levels for all but nine of treated dogs were in the same range as control dogs.
Animal Safety
Twenty-four (24) Beagle dogs were assigned to 4 groups (6 dogs/group) and were injected with placebo or 1X, 1.5X or 2X the recommended dose (volume) of Zeuterin™ in each testicle on days 0 and 14. The following adverse injection site reactions were displayed: mild discomfort when sitting down after the first injection (1X, 1.5X and 2X), difficulty walking 24 hours after the first injection (1 dog in the 2X group), swelling at 24-48 hours after the first and second injections (1X, 1.5X, 2X) and scrotal pain at 24 hours after the second injection (2X). There was an increase in resistance to the injection as the volume administered increased and as the size and consistency of the testicles changed in the 1X group after the first injection.
Six of the dogs developed scrotal irritation, dermatitis or necrosis post-Zeuterin™ injection. Two of these dogs developed mild scrotal irritation (1.5X group) or dermatitis (2X group) within 3 days of first and second injections, respectively. Four dogs (1X, 1.5X, 2X) developed more serious scrotal lesions (scrotal dermatitis with purulent discharge and necrosis), including one dog that required surgical castration due to necrosis of approximately one-half of the scrotal length. These more serious adverse reactions were observed from 24 hours to 8 days post-injection (in 2 dogs after the first injection and in 2 dogs after the second injection). These lesions occurred in 2 dogs that moved during the injection procedure, 1 dog with a pre-existing scrotal skin lesion and 1 dog where the injection was administered despite strong resistance to the injection. Housing conditions post-injection (wet, cement flooring) were also considered a contributing factor in the development of the scrotal lesions (See Warnings).
1 Normal Semen Values:
Sperm concentration: 200-1000 X 106/ejaculate
Semen volume: 1-40 mL/ejaculate
Spermatozoa motility >70% with progressive forward motility
2 Aspermia = No semen ejaculated
3 Azoospermia = No sperm in the ejaculate
4 Necrospermia = Sperm in the ejaculate are motionless/dead
5 Oligospermia = Sperm concentration less than 20 X 106 (for purposes of this field study)
Storage
Store at controlled room temperature 15-30°C (59-86°F).
How Supplied:
Zeuterin™ Injection is supplied in 3.5 mL sterile vials.
NADA 141-217 Approved by FDA
U.S. Patent Nos. 4,937,234; 5,070,080; 7,125,385; 7,276,535
Manufactured by: Oso BioPharmaceutical Manufacturing, LLC, Albuquerque, NM 87107
For: Ark Sciences, Inc., New York, NY 10019 U.S.A.
6-DAEJ-01
NAC No.: 1744000.0
50 SOUTH BUCKHOUT STREET, SUITE 203, IRVINGTON, NY, 10533
| Telephone: | 877-346-4664 | |
| Website: | www.arksciences.com |
 |
Every effort has been made to ensure the accuracy of the Zeuterin Injectable Solution information published above. However, it remains the responsibility of the readers to familiarize themselves with the product information contained on the US product label or package insert. |
Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
