The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
Vista Once SQ
This page contains information on Vista Once SQ for veterinary use.The information provided typically includes the following:
- Vista Once SQ Indications
- Warnings and cautions for Vista Once SQ
- Direction and dosage information for Vista Once SQ
Vista Once SQ
This treatment applies to the following species:BOVINE RHINOTRACHEITIS-VIRUS DIARRHEA-PARAINFLUENZA 3-RESPIRATORY SYNCYTIAL VIRUS-MANNHEIMIA HAEMOLYTICA-PASTEURELLA MULTOCIDA VACCINE
Modified Live Virus, Avirulent Live Culture
Cattle Vaccine
Product Description: The reconstituted vaccine product contains modified-live cultures of bovine rhinotracheitis (IBR) virus, bovine virus diarrhea (BVD) virus (Types 1 and 2); parainfluenza 3 virus (PI3), bovine respiratory syncytial virus (BRSV) and avirulent live cultures of Mannheimia haemolytica and Pasteurella multocida.
Vista Once SQ Indications
For the vaccination of healthy cattle, 3 months of age or older, as an aid in the prevention of respiratory disease caused by IBR, BVD (Type 2), and BRSV and as an aid in the control of disease caused by BVD (Type 1), PI3, Mannheimia haemolytica and Pasteurella multocida. Duration of Immunity (DOI) has been demonstrated to be at least 1 year for IBR and BVD (Types 1 & 2) and at least 16 weeks for Mannheimia haemolytica and Pasteurella multocida. Additionally, Vista® Once SQ is for the vaccination of healthy cows and heifers prior to breeding as an aid in the prevention of fetal infection, including persistently infected calves caused by BVD (Types 1 & 2); and as an aid in the prevention of persistently infected calves caused by BVD (Type 2); and as an aid in the reduction of abortion due to IBR. Reproductive Duration of Immunity (DOI) has been demonstrated to be at least 217 days for IBR, and at least 206 days for BVD (Types 1 & 2). Safe for use in pregnant heifers and cows or calves nursing pregnant cows provided the cows and heifers in the herd are vaccinated prior to breeding, within the previous 12 months, with any of the modified live IBR and BVD containing vaccine(s) in this product line.
Mixing Directions: 10 Doses - Rehydrate freeze dried vial of Vista® Once SQ with enclosed vial of diluent. Mix reconstituted vial well.
50 Doses - Rehydrate freeze dried vial of Vista® Once SQ with part of the accompanying vial of diluent using the transfer needle provided (see pictorial directions). Mix reconstituted vial well and transfer rehydrated vaccine into diluent vial using transfer needle. Remove transfer needle from former diluent vial and mix reconstituted vial well. Peel label from bottle of Vista® Once SQ and place on diluent vial containing all vaccine.
Use Directions: Inject 2.0 mL subcutaneously. Annual revaccination is recommended. A revaccination dose can be administered at more frequent intervals based upon individual farm disease risk assessment or any time epidemic conditions exist or are reported. Consult your veterinarian.
Mixing Instructions
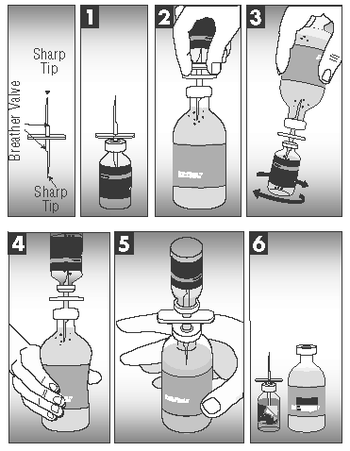
CAUTION: THE TRANSFER NEEDLE, INCLUDED IN THE CARTON PACKAGING, IS SHARP AND MAY CAUSE INJURY TO SELF OR ANIMALS IF NOT HANDLED OR DISPOSED OF PROPERLY.
1. Insert needle beyond breather valve into the vial of lyophilized vaccine to remove vacuum.
2. Turn the vial of lyophilized vaccine upside down and fully insert needle into the sterile diluent bottle. Be sure to clear cap with breather valve.
3. Turn attached vial and bottle so the vial of lyophilized vaccine is underneath the sterile diluent bottle and squeeze enough sterile diluent into the vial of lyophilized vaccine to rehydrate the vaccine. Mix well.
4. Turn attached bottles so rehydrated vaccine is above the sterile diluent bottle. Squeeze air into rehydrated vaccine vial.
5. Squeeze and release the sterile diluent bottle until all the solution in the rehydrated vaccine vial drains into the sterile diluent bottle.
6. Separate the vial of lyophilized vaccine and needle from the sterile diluent bottle. Using the tab, remove the back of the vaccine vial label. Place the separated label over the sterile diluent bottle label to accurately identify the newly created solution.
Cautions: Store at 2°-7°C (35°-45°F). Do not freeze. Use immediately after reconstitution; do not save partial contents. Burn the containers and all unused product. Use only in healthy cattle. Do not vaccinate within 21 days before slaughter. Fetal health risks associated with vaccination of pregnant animals with modified live vaccines cannot be unequivocally determined by clinical trials conducted for licensure. Management strategies based on vaccination of pregnant animals with modified live vaccines should be discussed with a veterinarian. If allergic reaction occurs, treat with epinephrine. Contains penicillin and streptomycin as preservatives.
FOR ANIMAL USE ONLY
Intervet Inc., Merck Animal Health, division of Intervet Inc., Omaha, NE 68103 USA
U.S. Veterinary License No. 165A
1-800-521-5767
For patent information:
http://www.merck.com/product/patent/home.html
|
|
|
Code |
|
|
10 Doses |
20 mL |
042978 |
128219-09 |
|
50 Doses |
100 mL |
006340 |
142232-08 |
CPN: 1047437.4
Intervet Inc.
2 GIRALDA FARMS, MADISON, NJ, 07940
| Customer Service: | 800-521-5767 | |
| Order Desk: | 800-648-2118 | |
| Technical Service (Companion Animal): | 800-224-5318 | |
| Technical Service (Livestock): | 800-211-3573 | |
| Fax: | 973-937-5557 | |
| Website: | www.merck-animal-health-usa.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |
Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
