VetScan Canine Borrelia Burgdorferi Antibody Test Kit
This page contains information on VetScan Canine Borrelia Burgdorferi Antibody Test Kit for veterinary use.The information provided typically includes the following:
- VetScan Canine Borrelia Burgdorferi Antibody Test Kit Indications
- Warnings and cautions for VetScan Canine Borrelia Burgdorferi Antibody Test Kit
- Direction and dosage information for VetScan Canine Borrelia Burgdorferi Antibody Test Kit
VetScan Canine Borrelia Burgdorferi Antibody Test Kit
This treatment applies to the following species:FOR THE QUALITATIVE DETECTION OF ANTIBODIES TO BORRELIA BURGDORFERI IN CANINE WHOLE BLOOD, PLASMA OR SERUM
For Veterinary Use Only
READ ALL INSTRUCTIONS BEFORE BEGINNING THE ASSAY
Intended Use
The VetScan Canine Borrelia Burgdorferi Antibody Test Kit is a visual and rapid test for the qualitative detection of antibodies to Borrelia burgdorferi in canine whole blood, serum or plasma. This test is for veterinary use only. B. burgdorferi is a spirochete that
causes Lyme disease in dogs, and some other animals. The disease is transmitted by ticks and it has a world-wide distribution. Clinical signs of Lyme disease include fever, arthritis and less commonly glomerulonephritis, uveitis, myocarditis and neurologic signs.
The VetScan Canine Borrelia Burgdorferi Antibody Test Kit uses peptides that bind antibodies elicited in response to certain Borrelia antigens in an amplified lateral flow sandwich assay. Antigen-coated colloidal gold particles bind to B. burgdorferi antibody in the sample. The bound antibody flows through the strip and is then captured by immobilized antigen on the test strip. The accumulation of the captured gold particle/antibody complex causes a color to become visible on the Test line (T). The intensity of the colored line is further enhanced by an amplification mechanism. A procedural Control line (C) will always appear whether the sample is positive or negative.
INSTRUCTION FOR USE
● This Test is for the detection of Borrelia Burgdorferi antibodies in canine samples.
● Refrigerated or frozen samples must be at room temperature 15° to 27°C (59° to 80°F) before running the assay-DO NOT HEAT.
● Whole canine blood collected in any type of EDTA, heparin, or citrate tubes may be used within one day of collection, provided no visual clotting has occurred. Do not freeze whole blood or use whole blood that has been frozen. If whole blood is not used within two hours of draw, store refrigerated.
● Serum or plasma, either fresh, previously frozen, or stored at 2° to 8°C (35° to 46°F), may be used in this test. Serum or plasma may be stored for use up to 7 days at 2° to 8°C (35° to 46°F). For longer storage, sample should be frozen at -20°C (-4°F) or colder.
● Previously frozen or older serum or plasma samples must be centrifuged at >1600g to remove any particulate material before use.
● Excessive hemolysis may obscure the results.
● EDTA, heparin, or ACD in plasma will not affect the results.
Precautions And Warnings
VetScan Canine Borrelia Burgdorferi Antibody Test Kit Caution
● Important: Do not remove Test Device from the pouch until ready for use.
● Test Device must be used as soon as possible after removing from pouch and within a maximum of 15 minutes.
● For veterinary use only.
● Do not use components after expiration date.
● The Test Device should be used in a horizontal position on a flat surface while the test is performed. The Test Device should not be moved or tilted during the test procedure.
● Use a separate Transfer Pipette for each test.
● The Chase Buffer is not interchangeable from serial (lot) to serial (lot).
● Do not use a Test Device from a pouch that is obviously torn or damaged.
● Do not use a Test Device if it appears cracked, broken, or otherwise damaged.
● The Kit Components must not be frozen.
STORAGE
● The Test Devices and Chase Buffer must be stored at 2° to 27°C (35° to 80°F) and never frozen.
● Test Devices and Chase Buffer are stable until expiration when stored at recommended temperatures.
KIT COMPONENTS
1. Test Devices
2. Lyme Chase Buffer Bottle
3. Transfer Pipettes
4. Instruction for Use
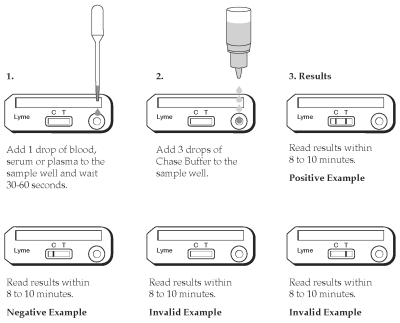
TEST PROCEDURE
1. Remove the Test Device from the protective pouch and place on a flat surface. Label the Test Device with the patient I.D. or control identification.
2. Gently mix the sample by inverting.
3. Using the Transfer Pipette provided, transfer one drop of sample (whole blood, serum or plasma) in to the sample well.
4. Let the sample absorb for 30-60 seconds.
5. Holding the Chase Buffer Bottle vertically, add 3 drops of the chase buffer into the sample well. Read the results within 8-10 minutes. High positive results may appear as soon as 1 minute, and low positive results may take up to 8-10 minutes to appear. Do not read results after 15 minutes. Colored lines which appear after 15 minutes are not diagnostic and should be ignored.
INTERPRETATION OF TEST RESULTS
Positive Results
The test is positive if two colored lines appear. One colored line will appear at the Test line (T) area and other in the Control line (C) area. Any intensity of the Test line (T) should be considered positive. Colored lines may be lighter or darker than each other.
Negative Results
The test is negative if only one line appears at the Control line (C) area.
Invalid Results
The test is invalid if no colored line appears at the Control line (C) area even if a colored line appears at the Test line (T) area. Colored lines that appear after 15 minutes are not diagnostic and should be ignored.
LYME TEST PROCEDURE
REFERENCES
Littman MP, Goldstein RE, Labato MA, Lappin MR, Moore GE. ACVIM small animal consensus statement on Lyme disease in dogs: diagnosis, treatment, and prevention. J Vet Intern Med. 2006 Mar-Apr;20(2):422-34.
READ ALL INSTRUCTIONS BEFORE BEGINNING THE ASSAY
Manufacturer
Abaxis, Inc., 3240 Whipple Road, Union City, CA 94587
800-822-2947
www.abaxis.com
Authorized Representative In The European Community
ABAXIS Europe GmbH, Bunsenstr. 9-11, 64347 Griesheim, Germany
+49 6155 780 210
U.S. Veterinary License No. 424
For patent information, see www.abaxis.com/about_us/patents
510-7020-1 Rev. B
Presentation: 25 test kit.
CPN: 1740000.2
Distributed by ZOETIS INC.
333 PORTAGE STREET, KALAMAZOO, MI, 49007
| Telephone: | 269-359-4414 | |
| Customer Service: | 888-963-8471 | |
| Website: | www.zoetis.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |
Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
