Quest Gel (Canada)
This page contains information on Quest Gel for veterinary use.The information provided typically includes the following:
- Quest Gel Indications
- Warnings and cautions for Quest Gel
- Direction and dosage information for Quest Gel
Quest Gel
This treatment applies to the following species:moxidectin
DIN 02231660
VETERINARY USE ONLY
Parasiticide
For Horses and Ponies
Introduction
QUEST® Gel contains moxidectin as its active ingredient. Moxidectin is a second generation endectocide of the milbemycin class of compounds that is highly effective, when administered at the recommended dosage of 400 µg per kg of body weight, against a broad range of economically important endoparasites of horses. Of particular interest are its indications as an aid in the treatment of parasitic infections caused by the arterial stages of S. vulgaris, as well as the developing, encysted mucosal larvae of cyathostomes (small strongyles) in horses and ponies. In addition, QUEST Gel, when used at the recommended dose, suppresses strongyle egg production for up to 84 days following a single administration, thereby reducing pasture contamination and providing a period of protection from reinfection for equines grazing that pasture.
Mode of action studies indicate that moxidectin eliminates parasites by interfering with chloride channel-mediated neurotransmission in the parasite, resulting in paralysis and elimination. The structural characteristics of the mammalian nervous system insure the safe use of moxidectin in horses and ponies.
Composition
QUEST Gel has been formulated in a highly palatable gel base for ease of administration. Each mL contains 20 mg of moxidectin (2% w/v); benzyl alcohol (4% w/v) has been added as a preservative.
Quest Gel Indications
QUEST Gel is indicated for the treatment of internal parasitic infections caused by the following parasites in healthy horses and ponies, 4 months of age or older:
Large Strongyles
Strongylus vulgaris (adults)
S. edentatus (adults and visceral, migratory stages)
Triodontophorus brevicauda (adults)
T. serratus (adults)
QUEST Gel is also indicated as an aid in the treatment of parasitic infections caused by the arterial stages of S. vulgaris.
Small Strongyles
Adult and luminal, larval (L4) stages of Cyathostomum spp., including Cyathostomum catinatum and C. pateratum;
Adult and luminal, larval (L4) stages of Cylicocyclus spp., including Cylicocyclus brevicapsulatus, C. elongatus, C. insigne, C. leptostomum, C. nassatus and C. radiatus;
Adult and luminal, larval (L4) stages of Cylicostephanus spp., including Cylicostephanus calicatus, C. goldi, C. longibursatus and C. minutus;
Adult and luminal, larval (L4) stages of Cylicodontophorus spp.;
Adult and luminal, larval (L4) stages of Gyalocephalus capitatus;
Adult stage of Coronocyclus spp., including Coronocyclus coronatus, C. labiatus and C. labratus;
Adult stage of Petrovinema poculatus;
Adult stage of Poteriostomum imparidentatum;
QUEST Gel is also indicated as an aid in the treatment of parasitic infections caused by the developing, encysted mucosal larvae (LL3 and L4) of cyathostomes. It is expected that the administration of QUEST Gel as per label directions can eliminate approximately 80% of the developing, encysted cyathostomes.
Ascarids (adults And L4)
Parascaris equorum
Pinworms (adults And L4)
Oxyuris equi
Hairworms (adults)
Trichostrongylus axei
Stomach Bots (2nd And 3rd Instars)
Gasterophilus nasalis
G. intestinalis
Integumentary Microfilariae
Onchocerca cervicalis
Large-mouthed Stomach Worms (adults And Gastric L4)
Habronema muscae
QUEST Gel, when used at the recommended dose, suppresses strongyle egg production for up to 84 days following a single administration, thereby reducing pasture contamination and providing a period of protection from reinfection for equines grazing that pasture.
Quest Gel Dosage And Administration
QUEST Oral Gel is specifically formulated as a palatable gel which is easily administered to horses and ponies.
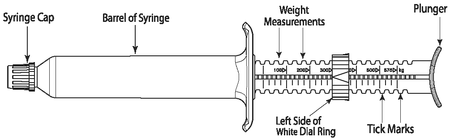
QUEST Gel is packaged in ready-to-use SURE-DIAL® syringes (see diagram of the SURE-DIAL® syringe below). The syringe is calibrated in 25 kg increments, up to 575 kg. This enables administration of the recommended dose level of 0.4 mg moxidectin/kg body weight by choosing a setting consistent with the animal’s weight.
How to set the dose
Since the dose is based on the weight of the animal, you need to use a scale or weight tape to find each animal’s weight before treating with QUEST Gel. Once the weight is known, set the dose of each horse or pony as follows.
1. Hold the syringe with the capped end pointing to the left so that you can see the weight measurements and tick marks (small black lines) as shown in the diagram below. Each tick mark relates to 25 kg body weight.
2. Turn the white dial ring until the left side of the ring lines up with the weight of the animal. In the diagram below the dial ring is set to dose a 300 kg animal.
How to give QUEST Gel to a horse or pony
1. Make sure there is no feed in the animal’s mouth.
2. Remove the cap from the end of the syringe. Save the cap for reuse.
3. Place the tip of the syringe inside the animal’s mouth at the space between the teeth.
4. Gently push the plunger until it stops, depositing the gel on the back of the tongue.
5. Remove the syringe from the animal’s mouth and raise the animal’s head slightly to make sure it swallows the gel.
6. Replace the syringe cap.
SURE-DIAL® Syringe
Treating a second horse or pony with the same syringe
If the animal you treat weighs less than 575 kg (1250+ lb), there will be gel left in the syringe. You can use this gel to treat other horses or ponies. To set the next dose, add the weight of the animal you want to treat to the dose setting already on the syringe. For example, if the syringe was first used to treat a 100 kg (220 lb) animal, the white dial ring is set on 100 kg. To treat a 250 kg (550 lb) animal next, move the white dial ring to the 350 kg marking (100+250=350). You need more than one syringe to treat horses weighing more than 575 kg (1250+ lb).
Each syringe of QUEST Gel may be used to treat more than one animal especially when dosing foals, ponies and growing and lighter breeds of horses. The table below will help to estimate the number of horses or ponies the contents of each syringe will treat.
|
Age |
Ponies |
Light Horses |
Heavy Horses |
|||
|
Approximate Weight |
No. ponies treated per syringe |
Approximate Weight |
No. horses treated per syringe |
Approximate Weight |
No. horses treated per syringe |
|
|
4 months |
75 (165) |
7 |
125 (275) |
4 |
175 (385) |
3 |
|
8 months |
100 (220) |
5 |
200 (440) |
2 |
250 (550) |
2 |
|
Mature |
200 (440) |
2 |
400 (880) |
1 |
500 (1100) |
1 |
If the animals are likely to be reinfected, a strategic parasite control program should be established: see Parasite Control Program below.
Parasite control program
For the best control of parasites, all horses and ponies should be included in a strategic treatment program, with particular care given to high performance animals, brood mares, stallions and foals. In foals, initial treatment is recommended at 4 months of age, after which they should be included in a routine retreatment program. Because QUEST Gel provides an aid in the control of developing, encysted cyathostomes (small strongyles), it may be particularly useful in reducing the frequency of dosing in a strategic treatment program. QUEST Gel, when used at the recommended dose, suppresses strongyle egg production for up to 84 days following a single administration, thereby reducing pasture contamination and providing a period of protection from reinfection for equines grazing that pasture. Consult your veterinarian for the diagnosis, prevention and treatment of parasitic infections in horses and ponies.
CAUTIONS
1. Extra care should be taken with foals to ensure that the correct dose is administered. Do not use in foals less than 4 months of age.
2. Quest Oral Gel has been formulated specifically for use in horses and ponies only. This product should not be used in other animal species as serious adverse reactions may result, such as death in dogs.
3. Overdoses, should they occur, may manifest themselves as transient depression, drowsiness and ataxia 8 to 24 hours post administration. Supportive treatment is not generally necessary and recovery is usually complete within 24 to 72 hours. There is no specific antidote for overdosing with moxidectin.
4. Do not mix this product with other veterinary medicinal preparations before administration.
5. Dispose of any containers and any residual content safely (e.g., bury or incinerate). Bury away from water courses, as free moxidectin may adversely affect fish and certain water borne organisms.
6. Some horses have experienced reactions with swelling and itching shortly after treatment. In most such cases, the horses have been diagnosed as carrying heavy infections of Onchocerca microfilariae, and it is assumed the reactions were the result of microfilariae dying in large numbers. Although the signs have resolved spontaneously in a few days, symptomatic treatment may be advisable. Consult your veterinarian should these symptoms persist.
WARNINGS
1. This drug is not to be administered to horses and ponies that are to be slaughtered for use in food.
2. Keep out of reach of children.
3. Avoid direct contact with skin and eyes.
Animal Safety
QUEST Gel can be safely administered at the recommended dose of 400 µg of moxidectin per kg of body weight to horses and ponies of all breeds, 4 months of age or older. Transient depression and/or ataxia has been observed occasionally in a small number of animals at three times the recommended dose level; however, it has been demonstrated that these effects diminish over time with minimal or no supportive therapy and result in no permanent cellular damage in the animal. The product has not been fully tested in young horses less than four months of age where multiple overdoses may result in reactions consistent with the known neurological mode of action for this class of compounds.
Reproductive safety studies demonstrate a wide margin of safety when the product is administered to cycling and pregnant mares, and breeding stallions as per label directions.
Storage
Store between 15 and 30°C. Keep from freezing.
Environmental Safety
Care should be taken to avoid release of significant volumes of moxidectin into either ground water or free-running water. Free moxidectin may adversely affect fish and certain water borne organisms. Moxidectin is readily and tightly bound to soil and rendered inactive over time. QUEST Gel syringes and their residual contents should be disposed of in an approved manner.
Packaging
QUEST Gel, DIN 02231660, is supplied as an individual, ready-to-use syringe which contains sufficient product to treat a 575 kg (1250+ lb) animal at the recommended dose of 400 µg of moxidectin per kg of body weight.
Zoetis is a trademark and Quest and SURE-DIAL are registered trademarks of Zoetis or its licensors, used under license by Zoetis Canada Inc.
Zoetis Canada Inc., Kirkland QC H9H 4M7
2202-11-0
P1142-652CA/02-15
CPN: 1198400.2
16,740 TRANS-CANADA HIGHWAY, KIRKLAND, QC, H9H 4M7
| Order Desk: | 800-663-8888 | |
| Technical Services Canada: | 800-461-0917 | |
| Technical Services USA: | 800-366-5288 | |
| Website: | www.zoetis.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |
Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
