Palladia Tablets (15 mg) (Canada)
This page contains information on Palladia Tablets (15 mg) for veterinary use.The information provided typically includes the following:
- Palladia Tablets (15 mg) Indications
- Warnings and cautions for Palladia Tablets (15 mg)
- Direction and dosage information for Palladia Tablets (15 mg)
Palladia Tablets (15 mg)
This treatment applies to the following species: Company: Zoetis
Company: Zoetis
DIN 02338548 (10 mg)
DIN 02338556 (15 mg)
DIN 02338564 (50 mg)
toceranib phosphate tablets
Veterinary Use Only
Anti-neoplastic for dogs
Palladia Tablets (15 mg) Indications
For the treatment of non-resectable Patnaik grade II (intermediate grade) or III (high grade) recurrent cutaneous mast cell tumors with or without regional lymph node involvement in dogs.
Note: PALLADIA tablets should be used only after carefully reading the Package Insert.
Dosage and Administration
Administer an initial dosage of 3.25 mg/kg body weight orally every other day. Dose reductions of 0.5 mg/kg (to a minimum dose of 2.2 mg/kg every other day) and dose interruptions (cessation of PALLADIA tablets for up to two weeks) may be utilized, if needed, to manage adverse reactions (see Dose Modification Based on Toxicity Observed Table and Cautions). Adjust dose based on approximately weekly veterinary assessments for the first 6 weeks and approximately every 6 weeks, thereafter. PALLADIA tablets may be administered with or without food. Do not split tablets.3.25 mg/kg Dose Chart
|
Dog Body Weight |
|
Number of Tablets |
||
|
Kilograms |
Dose |
10 mg |
15 mg |
50 mg |
|
5.0 - 5.3 |
15 mg |
|
1 |
|
|
5.4 - 6.9 |
20 mg |
2 |
|
|
|
7.0 - 8.4 |
25 mg |
1 |
1 |
|
|
8.5 - 10.0 |
30 mg |
|
2 |
|
|
10.1 - 11.5 |
35 mg |
2 |
1 |
|
|
11.6 - 13.0 |
40 mg |
1 |
2 |
|
|
13.1 - 14.6 |
45 mg |
|
3 |
|
|
14.7 - 16.1 |
50 mg |
|
|
1 |
|
16.2 - 17.6 |
55 mg |
1 |
3 |
|
|
17.7 - 19.2 |
60 mg |
1 |
|
1 |
|
19.3 - 20.7 |
65 mg |
|
1 |
1 |
|
20.8 - 23.0 |
70 mg |
2 |
|
1 |
|
23.1 - 26.9 |
80 mg |
|
2 |
1 |
|
27.0 - 29.9 |
95 mg |
|
3 |
1 |
|
30.0 - 32.3 |
100 mg |
|
|
2 |
|
32.4 - 34.6 |
110 mg |
1 |
|
2 |
|
34.7 - 36.1 |
115 mg |
|
1 |
2 |
|
36.2 - 38.4 |
120 mg |
2 |
|
2 |
|
38.5 - 43.0 |
130 mg |
|
2 |
2 |
|
43.1 - 47.6 |
150 mg |
|
|
3 |
|
47.7 - 49.9 |
160 mg |
1 |
|
3 |
|
50.0 - 51.5 |
165 mg |
|
1 |
3 |
The number of tablets required for dogs above 51.5 kg body weight should be calculated based on the 3.25 mg/kg dosage regime.
|
Dose Modification Based on Toxicity Observed |
|
|
Toxicity Grade |
Dose Adjustment |
|
Anorexia |
|
|
<50% food intake ≥2 days |
Stop treatment and institute dietary modification ± supportive care until food intake improves, then decrease dose by 0.5 mg/kg |
|
Diarrhea |
|
|
<4 watery stools/day for <2 days |
Maintain dose level and institute supportive care |
|
≥4 watery stools/day or ≥2 days |
Stop treatment until formed stools and institute supportive care. When dosing is resumed, decrease dose by 0.5 mg/kg |
|
Gastrointestinal Bleeding |
|
|
Fresh blood in stool or black tarry stool for >2 days or frank haemorrhage or blood clots in stool |
Stop treatment and institute supportive care until resolution of all clinical signs of blood in stool, then decrease dose by 0.5 mg/kg |
|
Neutropenia (neutrophil count) |
|
|
>1x109/L |
Maintain dose level |
|
≤1x109/L or neutropenic fever or infection |
Stop treatment until >1x109/L and clinical signs normal, then decrease dose by 0.5 mg/kg |
|
Thrombocytopenia (platelet count) |
|
|
>1x1011/L |
Maintain dose level |
|
≤1x1011/L |
Stop treatment until >1x1011/L, then decrease dose by 0.5 mg/kg |
|
Anemia (hematocrit) |
|
|
>26% |
Maintain dose level |
|
≤26% |
Stop treatment until >26%, then decrease dose by 0.5 mg/kg |
|
Hypoalbuminemia (albumin) |
|
|
Albumin <15 g/L |
Stop treatment until >25 g/L and clinical signs are normal, then decrease dose by 0.5 mg/kg |
|
Hepatic Toxicity (ALT, AST) |
|
|
>1X - 3X upper normal limit |
Maintain dose level; stop hepatotoxic drugs, if used |
|
>3X upper normal limit |
Stop treatment until ≤3X upper normal limit, stop hepatotoxic drugs if used, then decrease dose by 0.5 mg/kg |
|
Hepatic Toxicity (total bilirubin) |
|
|
17.1-34.2 µmol/L |
Maintain dose level; stop hepatotoxic drugs, if used |
|
>34.2 µmol/L |
Stop treatment until ≤34.2 µmol/L, stop hepatotoxic drugs if used, then decrease dose by 0.5 mg/kg |
|
Renal Toxicity (creatinine) |
|
|
<176 µmol/L |
Maintain dose level |
|
≥176 µmol/L |
Stop treatment until <176 µmol/L, then decrease dose by 0.5 mg/kg |
|
Concurrent anemia, azotemia, hypoalbuminemia and hyperphosphatemia |
|
|
Stop treatment for 1 to 2 weeks until values have improved and albumin >25 g/L, then decrease dose by 0.5 mg/kg |
|
Contraindications
- Do not use in breeding, pregnant or lactating dogs.
- Do not use if there is evidence of gastrointestinal bleeding.
CAUTIONS:
The presence of systemic mast cell tumour prior to treatment may predispose a dog to clinically significant mast cell degranulation with possible severe systemic side effects when treated with PALLADIA tablets. Attempts should be made to rule out systemic mastocytosis prior to initiation of treatment with PALLADIA tablets.
PALLADIA tablets have been associated with severe diarrhea or GI bleeding that requires prompt treatment. Dose interruptions and dose reductions may be needed depending upon the severity of clinical signs. Use non-steroidal anti-inflammatory drugs with caution in conjunction with PALLADIA tablets due to an increased risk of gastrointestinal ulceration or perforation.
PALLADIA tablets are metabolized in the liver. Co-administration of PALLADIA tablets with strong inhibitors of the CYP3A family (e.g., azole antifungals, macrocyclic lactone antibiotics) may increase toceranib concentrations. The effect of concomitant medications that may inhibit the metabolism of PALLADIA tablets has not been evaluated. Drug compatibility should be monitored in dogs requiring concomitant medications.
The safe use of PALLADIA tablets has not been evaluated in dogs less than 24 months of age or weighing less than 5 kg.
PALLADIA tablets may cause vascular dysfunction, which can lead to edema and thromboembolism, including pulmonary thromboembolism. Discontinue drug until clinical signs and clinical pathology have normalized. To assure vasculature homeostasis, wait at least 3 days after stopping drug before performing surgery (See Adverse Reactions).
Serious and sometimes fatal gastrointestinal complications, including gastrointestinal perforation, have occurred in dogs treated with PALLADIA tablets (See Adverse Reactions). If gastrointestinal ulceration is suspected, stop drug administration and treat appropriately.
Other treatment modalities such as surgery and radiation should be considered as the first lines of treatment for localized disease.
PALLADIA tablets have not been evaluated in dogs receiving concurrent chemotherapy.
Warnings
NOT FOR USE IN HUMANS. KEEP OUT OF REACH OF CHILDREN. Keep children away from feces, urine, or vomit of treated dogs. Wash hands with soap and water after administering PALLADIA tablets.
Pregnant women, women who may become pregnant, or nursing mothers should pay special attention to the handling procedures detailed below as PALLADIA tablets belong to a class of agents that may cause harm to the unborn baby. PALLADIA tablets prevent the formation of new blood vessels in tumours. In a similar manner, PALLADIA tablets may affect blood vessel formation in the developing fetus and may harm an unborn baby (cause birth defects). For pregnant women, accidental ingestion of PALLADIA tablets may have adverse effects on pregnancy.
If PALLADIA tablets are accidentally ingested, seek medical advice immediately. It is important to show the treating physician a copy of the package insert or label. In cases of accidental human ingestion of PALLADIA tablets, gastrointestinal discomfort, including vomiting or diarrhea, may be experienced.
Handling procedures:
Do not split or break tablets to avoid disrupting the protective film coating. PALLADIA tablets should be administered immediately after they are removed from the bottle.
Protective gloves should be worn if handling broken or moistened tablets.
If the dog spits out the PALLADIA tablet, the tablet will be moistened and should be handled with protective gloves. If the PALLADIA tablet is “hidden” in food, make sure that the dog has eaten the entire dose.
Because PALLADIA tablets are present in the stool, urine and vomit of dogs under treatment, wear protective gloves to clean up after the treated dog. Place the stool, feces or vomit, and any disposable towels used to clean up in a plastic bag, which should be sealed for general household disposal. This will minimize the potential for exposure to people in contact with the trash.
Do not wash any items soiled with stool, urine or vomit from treated dog with other laundry.
The material safety data sheet (MSDS) contains more detailed occupational safety information.
To report adverse reactions in users or to obtain a copy of the MSDS for this product, call 1-800-461-0917.
Adverse Reactions
A U.S. pivotal clinical field study comprised of a 6-week masked phase followed by an open label phase evaluated the safety and effectiveness of PALLADIA tablets in 151 client-owned dogs that had Patnaik grade II or III recurrent cutaneous mast cell tumours with or without regional lymph node involvement. The most common adverse reactions reported during the masked phase are summarized in Table 1; those reported during the entire study (masked phase combined with the open-label phase) are summarized in Table 2.
Table 1. Summary of the most common adverse reactions during the masked phase
|
|
Placebo (n = 64) |
PALLADIA (n = 87) |
||
|
Adverse Reaction |
Any Grade1 |
Grade 3 or 41 |
Any Grade1 |
Grade 3 or 41 |
|
Diarrhea |
26.6% |
3.1% |
46.0% |
6.9% |
|
Anorexia |
31.3% |
6.3% |
39.1% |
6.9% |
|
Lethargy |
29.7% |
3.1% |
35.6% |
4.6% |
|
Vomiting |
32.8% |
6.3% |
32.2% |
9.2% |
|
Lameness |
9.4% |
0.0% |
17.2% |
0.0% |
|
Weight loss |
3.1% |
0.0% |
14.9% |
1.1% |
|
Musculoskeletal disorder |
6.3% |
0.0% |
11.5% |
1.1% |
|
Blood in stool/GI bleed/Hemorrhagic diarrhea |
3.1% |
0.0% |
12.6% |
2.3% |
|
Dehydration |
4.7% |
0.0% |
9.2% |
2.3% |
|
Dermatitis |
9.4% |
1.6% |
9.2% |
0.0% |
|
Pruritus |
4.7% |
0.0% |
9.2% |
0.0% |
|
Tachypnea |
4.7% |
0.0% |
8.0% |
1.1% |
|
Localized pain |
4.7% |
0.0% |
8.0% |
0.0% |
|
Nausea |
3.1% |
0.0% |
8.0% |
1.1% |
|
General pain |
4.7% |
1.6% |
6.9% |
0.0% |
|
Polydipsia |
7.8% |
0.0% |
6.9% |
0.0% |
|
Pyrexia |
3.1% |
0.0% |
5.7% |
2.3% |
|
Flatulence |
3.1% |
0.0% |
5.7% |
0.0% |
|
Laboratory Abnormality |
Any Grade2 |
Grade 3 or 42 |
Any Grade2 |
Grade 3 or 42 |
|
Neutropenia |
6.3% |
0.0% |
46.0% |
0.0% |
|
Thrombocytopenia |
20.3% |
0.0% |
24.1% |
0.0% |
|
Increased Alanine Aminotransferase |
21.9% |
4.7% |
24.1% |
1.1% |
|
Decreased Hematocrit |
7.8% |
0.0% |
5.7% |
3.4% |
|
Hypoalbuminemia |
7.8% |
0.0% |
12.6% |
0.0% |
|
Hyperbilirubinemia |
1.6% |
1.6% |
5.7% |
0.0% |
|
Increased Creatinine |
4.7% |
0.0% |
5.7% |
0.0% |
|
Urinary tract infection |
1.6% |
0.0% |
5.7% |
0.0% |
1 Investigators assigned severity grade of 1, 2, 3 or 4 (1 - least severe; 4 - most severe).
2 Grading of laboratory abnormalities was based on the National Cancer Institute’s Common Toxicity Criteria Guideline adapted for canines (1 - least severe; 4 - most severe).
The mean time on study during the masked phase was 37.0 days for PALLADIA tablets-treated dogs (median, 42.0 days) and 27.6 days for placebo-treated dogs (median, 21.0 days); no adjustments were made in the statistical comparisons for this disparity.
Table 2. Summary of the most common adverse reactions during the study (masked phase combined with the open-label phase)
|
|
PALLADIA (n = 145)1 |
|
|
Adverse Reactions |
Any Grade2 |
Grade 3 or 42 |
|
Diarrhea |
58.6% |
8.3% |
|
Anorexia |
49.7% |
8.3% |
|
Vomiting |
47.6% |
9.7% |
|
Lethargy |
39.3% |
4.1% |
|
Lameness |
22.8% |
0.0% |
|
Weight loss |
21.4% |
2.8% |
|
Blood in stool/GI bleed/Hemorrhagic diarrhea |
18.6% |
2.8% |
|
Dehydration |
15.2% |
2.1% |
|
Pruritus |
12.4% |
0.0% |
|
Pigmentation disorder |
11.7% |
0.0% |
|
Dermatitis |
11.0% |
0.0% |
|
Musculoskeletal disorder |
11.0% |
0.0% |
|
General pain |
8.3% |
0.0% |
|
Otitis externa |
8.3% |
0.0% |
|
Tachypnea |
8.3% |
0.0% |
|
Nausea |
7.6% |
1.4% |
|
Polydipsia |
7.6% |
0.0% |
|
Pyrexia |
6.9% |
2.8% |
|
Arthritis |
6.2% |
0.0% |
|
Localized edema |
6.2% |
0.0% |
|
Bacterial skin infection |
5.5% |
0.0% |
|
Conjunctivitis |
5.5% |
0.0% |
|
Laboratory Abnormality |
Any Grade3 |
Grade 3 or 43 |
|
Neutropenia |
44.8% |
1.4% |
|
Hypoalbuminemia |
28.3% |
1.4% |
|
Thrombocytopenia |
28.3% |
2.1% |
|
Increased Alanine Aminotransferase |
27.6% |
4.1% |
|
Decreased Hematocrit |
11.0% |
2.8% |
|
Increased Creatinine |
13.8% |
1.4% |
|
Hyperbilirubinemia |
6.9% |
0.0% |
|
Urinary Tract Infection |
7.6% |
0.0% |
1All dogs received at least 1 dose of PALLADIA tablets.
2Investigators assigned severity grade of 1, 2, 3 or 4 (1 - least severe; 4 - most severe).
3Grading of laboratory abnormalities was based on the National Cancer Institute’s Common Toxicity Criteria Guideline adapted for canines (1 - least severe; 4 - most severe).
Other adverse events were reported but occurred in < 5% of dogs. Any individual dog may have had multiple adverse events.
The duration of treatment with PALLADIA tablets ranged from 2 to 812 days (mean, 144 days; median, 68 days).
There were 5 deaths during this study that were possibly drug related. Pathology findings generally revealed evidence of vascular dysfunction, including pulmonary thromboembolism (post-operative); multi-organ failure associated with vasculitis and thrombosis; vascular thrombosis with disseminated intravascular coagulopathy (DIC), and pancreatitis; and vasculitis with DIC. One dog died secondary to gastric perforation; the duration of treatment with PALLADIA tablets was 221 days, and there was no evidence of mast cell tumour at necropsy. These deaths occurred in the presence or absence of gross-disease; treatment durations ranged from 18 to 221 days.
The relationship of the following deaths to drug is unknown. One dog, first treated for 3 weeks with a placebo, died of unknown cause 7 days after initiation of PALLADIA tablets therapy. Another dog died of unknown cause 92 days after initiation of PALLADIA tablets therapy. No necropsy was conducted in either dog.
Twenty seven dogs developed some form of gastrointestinal bleeding, with 3.4% of dogs having severe bleeding. One dog developed gastric ulceration, which was possibly drug related. Three dogs died from gastric (1 dog) or duodenal (2 dogs) perforations during the study. One dog with a duodenal perforation received only 1 dose of the study drug and, therefore, was not considered drug related.
Seven dogs developed nasal depigmentation within the first few weeks of treatment. Eleven dogs developed coat color or skin changes during the study. Two of these dogs had complete coat color changes from fawn to white and from deep red to blonde. Seven dogs experienced alopecia. Two dogs had severe necrosis of skin overlying the mast cell tumour.
There is a drug related effect on body weight: 20.0% of dogs had loss of condition (>13% weight loss) in the masked plus open-label phase attributable to drug. Five of these dogs had >25% weight loss.
Three dogs had seizure-like activity while on study drug. It can not be determined if these were drug related.
Two dogs developed epistaxis that was not associated with thrombocytopenia. Another dog developed epistaxis with concurrent disseminated intravascular coagulopathy.
In the masked and open-label phases of the study, more male dogs (25/61, 41%) had a grade 3 or 4 adverse events than female dogs (25/84, 29.8%).
Clinical Pharmacology
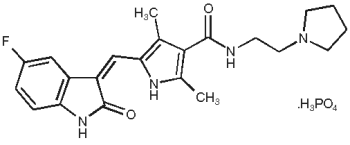
Description: PALLADIA, a multi-kinase inhibitor targeting several receptor tyrosine kinases (RTK), is the phosphate salt of toceranib. The empirical formula is C22H25FN4O2•H3O4P, and the molecular weight is 494.46. The chemical name is (Z)-5-[(5-Fluoro-2-oxo-1,2-dihydro-3H-indol- 3-ylidene)methyl]-2,4-dimethyl-N-(2-pyrrolidin-1-ylethyl)-1H-pyrrole-3-carboxamide phosphate. Toceranib phosphate is a small molecule with an indolinone chemical structure.
The chemical structure of toceranib phosphate is:
Mechanism of Action: Toceranib is a small molecule that belongs to a class of agents that has both direct antitumour and antiangiogenic activity. In non-clinical pharmacology studies, toceranib selectively inhibited the tyrosine kinase activity of several members of the split kinase receptor tyrosine kinase (RTK) family, some of which are implicated in tumour growth, pathologic angiogenesis, and metastatic progression of cancer. Toceranib inhibited the activity of Flk-1/KDR tyrosine kinase (vascular endothelial growth factor receptor, VEGFR2), platelet derived growth factor receptor (PDGFR), and stem cell factor receptor (Kit) in both biochemical and cellular assays. Toceranib has been shown to exert an antiproliferative effect on endothelial cells in vitro. Toceranib treatment can induce cell cycle arrest and subsequent apoptosis in tumour cell lines expressing activating mutations in the split kinase RTK, c-kit. Canine mast cell tumour growth is sometimes driven by activating mutations in c-kit.
Other compounds in the antiangiogenesis class of antineoplastic agents are known to increase embryolethality and fetal abnormalities. As angiogenesis is a critical component of embryonic and fetal development, inhibition of angiogenesis following administration of PALLADIA tablets should be expected to result in adverse effects on the pregnancy in the bitch.
Pharmacokinetics: Following intravenous administration, the pharmacokinetics of toceranib is characterized by a very large volume of distribution (>20 L/kg, indicating partitioning into tissues), a terminal elimination half-life of about 16 hrs, and a clearance of >1 L/hr/kg. With a regimen of 3.25 mg free base equivalent (fbe)/kg doses of toceranib administered by tablet orally every other day for 2 weeks (7 doses), the pharmacokinetic parameters of toceranib in plasma in healthy Beagle dogs (between 7.2 - 12.5 kg) are shown in the following chart.
|
Pharmacokinetic Parameters (Mean + 1SD) |
Total (n=11; 6M, 5F) |
Total (n=10; 5M, 5F) |
|
Elimination half-life, t1/2 (h) |
16.4 ± 3.6 |
17.2 ± 3.9 |
|
Time to maximum plasma concentration, Tmax (h) |
5.3 ± 1.6 |
6.2 ± 2.6 |
|
Maximum plasma concentration, Cmax (ng/mL) |
86 ± 22 |
109 ± 41 |
|
1,2Cmin (ng/mL) |
12.7 ± 6.0 |
18.7 ± 8.3 |
|
1Area under the plasma concentration time-curve, AUC0-48 (ng•h/mL) |
1833 ± 508 |
2635 ± 939 |
1Dose-normalized value (adjusted to 3.25 mg/kg dose)
2Cmin is the concentration at 48 hr post-dose, which corresponds to the dose interval.
Oral bioavailability of toceranib is 77%. Fed or fasted state does not significantly affect the absorption of toceranib. Toceranib is highly protein bound at 91% to 93%.
It should be noted that despite the homogeneity of subjects included in this study, large between subject variability was observed. Regardless of the route of administration, linear pharmacokinetics has been observed at doses up to 5 mg/kg twice daily. Using an in vitro hepatocyte and liver microsome test system, the Z isomer was found to be metabolized to the N-oxide derivative of toceranib in dogs, humans, cats, and rats. Although a small gender difference was observed in the in vitro study (81% conversion in male dogs, 56% conversion in female dogs) no differences in toceranib phosphate pharmacokinetics was observed in vivo. The effects of renal impairment, hepatic impairment or breed on the pharmacokinetics of toceranib have not been investigated.
ANIMAL SAFETY:
A laboratory study was conducted with PALLADIA tablets in healthy adult Beagle dogs to describe the toxicity associated with the anticipated dose range. PALLADIA tablets were administered orally to 20 male and 20 female adult dogs (approximately 2 years of age) at doses of 0 mg/kg (placebo, 12 dogs), 2 mg/kg (0.5X, 8 dogs), 4 mg/kg (1X, 12 dogs), or 6 mg/kg (1.5X, 8 dogs) once every other day for 13 consecutive weeks without dose interruption. PALLADIA tablets caused weight loss, decreased feed consumption, pancreatic, gonadal, adrenal, muscle, and hematopoietic changes with variable dose relationship.
A group decrease in feed consumption was apparent in the 1.5X group compared to placebo, with the largest decrease in means occurring at Day 35. Group differences in body weight were apparent at both 1X and 1.5X compared to placebo with the animals reaching a lower, stable body weight around Day 31. Findings of sporadic dose related lameness were greater in all treatment groups as compared to placebo, with the 1.5X group demonstrating the highest incidence rate and observed almost exclusively in the hind limbs. Stiffness, weakness, and pain in limbs were noted to occur almost exclusively in the 1.5X treatment group. Redness of oral mucosa was observed, but was considered of doubtful relationship to dosing. In the 1.5X group, 3/8 dogs showed blood in their feces; two of these dogs were ultimately euthanized for treatment-related clinical toxicities (see below).
Hematology analyses showed a decrease in erythrocyte count (1X and 1.5X) and drop in reticulocyte count, although the reticulocytes continued to partially offset further decreases in erythrocyte count. White blood cell counts were significantly lower across the study in all treated groups compared to placebo, primarily due to 20 - 40% decrease in neutrophils. Lymphocytes were less affected, especially at the low dose. Eosinophils and basophils showed marked, persistent decreases. Monocytes were not affected. Platelet counts increased slightly in the mid- and high-dose groups. Fibrinogen (1X and 1.5X) and globulin (1.5X) concentrations increased.
Increases were observed in aspartate aminotransferase (less than 100% in 1.5X), lactate dehydrogenase (41% in 1.5X), creatine kinase (54% in 1.5X), and alkaline phosphatase (31% in 1.5X). An increase was identified in amylase in two males, one at 1X and the other at 1.5X. A minor decrease in serum potassium and a minor increase in serum phosphorus concentrations were noted at 1.5X. A minor decrease was identified in urine pH, but other urinalysis parameters were normal.
Treatment-related microscopic changes included slight to marked reduction in cellularity of sternal and femoral bone marrow, more prominent in females. There was a corresponding mild extramedullary hematopoiesis, mainly erythropoiesis, in the spleen. In the pancreas, dose-related slight to moderate acinar degranulation, characterized by diffuse loss of zymogen granules, occurred in both sexes at all doses, but more markedly in females. In the adrenal glands, minimal cortical congestion/hemorrhage occurred in both sexes at all doses, with suggestive dose-relationship. Adrenal cortical vacuolation was noted with low frequency in all groups, but with slightly increased severity in females. Dose related changes were noted in reproductive organs of both sexes. Males showed a dose-related germ cell depletion, tubular vacuolation, and reductions in numbers of mature spermatozoa. In females, ovaries showed a reduced incidence of mature/regressing corpora lutea, and an increased incidence of small follicles.
Two dogs (one male one female) in the 1.5X group were euthanized for treatment-related clinical toxicities. Onset of the terminal syndrome was marked by markedly reduced feed intake and melena. Over the following 9 days, the decreased feed intake progressed to near-complete anorexia, and hematochezia appeared. Weight loss, lameness and weakness were observed. In addition to the microscopic findings described in animals surviving to the end of the study, these dogs showed lymphoid depletion in lymph nodes and gut-associated lymphatic tissues and mild to marked gastrointestinal lesions that included necrotizing vasculitis of small vessels. These two dogs also had lesions in kidneys and in other tissues that may have been due to their moribund clinical condition at euthanasia.
Effectiveness
The effectiveness and safety of PALLADIA tablets for the treatment of mast cell tumours was evaluated in a randomized, placebo-controlled, double-masked, multicenter clinical field study. The purpose of this study was to evaluate the effectiveness and safety of PALLADIA tablets in the treatment of mast cell tumours in dogs that had recurrent measurable disease after surgery and to evaluate objective response (complete or partial response). PALLADIA tablets treatment was compared to placebo treatment using response rates at the end of the 6-week masked phase. Response rates were determined using the U.S. National Cancer Institute’s Response Evaluation Criteria in Solid Tumors Guideline, which was modified specifically for the evaluation of canine mast cell tumours.
One-hundred-fifty-three dogs were randomly assigned to treatment with either 3.25 mg/kg PALLADIA tablets (n = 88) or placebo (n = 65) orally, every other day for 6 weeks or, until disease progression or withdrawal from the study for another cause. Treatment was unmasked at the time of disease progression: dogs receiving placebo were then offered crossover to open label PALLADIA tablets; dogs receiving PALLADIA tablets were discontinued from the study.
Treatment in the extended therapy phase could continue for eligible dogs until seven months after the last dog enrolled onto the blinded phase of the study. Dogs treated with PALLADIA tablets in the open label phase (n=111) included 58/65 dogs treated with placebo and 53/88 dogs treated with PALLADIA tablets in the blinded phase. Dogs were required to have Patnaik grade II or III recurrent cutaneous mast cell tumours with or without regional lymph node involvement. At least 1 tumour had to be at least 20 mm in diameter. Dogs had a limit of 1 completed radiation protocol and a limit of 1 prior systemic chemotherapy regimen. Dogs with evidence of systemic mast cell tumour were excluded. Treatment with systemic corticosteroids during the study or within 14 days prior to study initiation was not permitted. If needed to manage adverse reactions, dose interruptions (cessation of PALLADIA tablets for up to 2 weeks) were prescribed and/or dosage was reduced to as low as 2.2 mg/kg. The effectiveness analysis showed a statistically significant advantage for PALLADIA tablets over placebo in the primary effectiveness endpoint of objective response measured at the end of the six-week blinded phase.
Mast Cell Tumor - Primary Effectiveness Endpoint Results
|
Effectiveness Parameter |
Placebo (n = 63) |
PALLADIA tablets (n = 86) |
P-value |
|
Objective Response Rate * |
7.9% |
37.2% |
<0.001 |
* The difference in objective response rate between groups was not significantly associated with tumor burden (presence vs. absence of regional lymph node involvement) or tumor grade (P > 0.05).
The objective response is the total of complete responses and partial responses. A complete response was noted for 8.1%, and a partial response was noted for 29.1%.
Sixty-two of 145 dogs (43%) had an objective response during the combined blinded and open label phases. The median duration of objective response was 84 days (12.0 weeks; CI, 6.7, 18.3).
The median time to progression (TTP) for placebo-treated and PALLADIA tablets-treated dogs during the blinded phase was 3.0 and >6.0 weeks, respectively; this difference was significant (P<0.0001). The median TTP for dogs treated with PALLADIA tablets in the blinded phase (n=86) and the median TTP for all dogs that received at least one dose of PALLADIA tablets in the combined blinded and open label phases (n=145) was 9.1 and 11.9 weeks, respectively.
The change from baseline in health-related quality of life (HRQL) scores using an exploratory HRQL questionnaire was not statistically different between PALLADIA tablets-treated and placebo-treated groups at the end of the blinded phase (P=0.770). PALLADIA tablets-treated dogs that responded (complete or partial response) to treatment had a significantly higher HRQL score at the end of the blinded phase than those that did not respond (+2 vs. -7, respectively; P=0.030).
Of 145 dogs treated with at least one dose of PALLADIA tablets during the combined blinded and open label phases, dogs with c-kit positive tumours were more likely to respond than dogs with c-kit negative tumours (69% vs. 37%, respectively; P=0.0008; odds ratio 4.47). Of the 145 dogs, 20% had c-kit positive tumours. c-kit mutation status was not significantly associated with duration of objective response (P=0.239).
During the study, PALLADIA tablets were administered concomitantly with other medications such as antimicrobials, H-2 receptor blockers, antihistamines, anti-emetics, non-steroidal anti-inflammatory drugs, locally-acting antiulcer medications, opiate gastro-intestinal motility modifiers, opioids, vaccines, anthelmintics, antiparasitics, and topical/ophthalmic/otic corticosteroid preparations. During the open label phase only, 5 dogs received a brief course of short-acting corticosteroids.
Storage
Store between 15 and 30°C.PRESENTATION: Each color-coded PALLADIA tablet contains 10 mg, 15 mg, or 50 mg of toceranib (as toceranib phosphate) per tablet. The tablets are packaged in 30 count bottles. Each tablet is marked with the tablet strength on one side and blank on the other.
Zoetis is a trademark and Palladia is a registered trademark of Zoetis or its licensors, used under license by Zoetis Canada Inc.
Zoetis Canada Inc., Kirkland QC H9H 4M7
1690-11-2
PAA115520
CPN: 1198355.3
16,740 TRANS-CANADA HIGHWAY, KIRKLAND, QC, H9H 4M7
| Order Desk: | 800-663-8888 | |
| Technical Services Canada: | 800-461-0917 | |
| Technical Services USA: | 800-366-5288 | |
| Website: | www.zoetis.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |
Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
