The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
Intra-Trac-II
This page contains information on Intra-Trac-II for veterinary use.The information provided typically includes the following:
- Intra-Trac-II Indications
- Warnings and cautions for Intra-Trac-II
- Direction and dosage information for Intra-Trac-II
Intra-trac-ii
This treatment applies to the following species:Canine Parainfluenza-bordetella Bronchiseptica Vaccine
Modified Live Virus, Avirulent Live Culture
Intra-Trac®-II vaccine is recommended for use as an aid in the prevention of disease associated with canine parainfluenza virus and Bordetella bronchiseptica infection in healthy dogs 3 weeks of age or older. These agents have been implicated as playing a role in the etiology of the condition known as canine kennel cough (infectious canine cough).
When To Vaccinate
Vaccinate dogs at 3 weeks of age or older. Annual revaccination is recommended.
Preparation Of The Vaccine
1. Rehydrate live vaccine to desired volume with the accompanying Sterile Diluent*.
2. Draw the vaccine back into the syringe.
3. Remove needle from syringe.
4. Apply nasal applicator tip to syringe.
How To Vaccinate
*Standard dose is 1 mL administered intranasally. Vaccine may be rehydrated with less than 5 mL of diluent to minimize discomfort when vaccinating small dogs and puppies. For five 1 mL doses, rehydrate vaccine with 5 mL sterile diluent or for five 0.5 mL doses, rehydrate vaccine with 2.5 mL sterile diluent. Instill the rehydrated vaccine into one or both nostrils of five dogs as illustrated below.
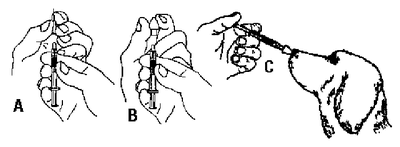
Intra-Trac-II Caution
1. This product is designed for intranasal use only with the enclosed applicators. Systemic reactions resulting from inadvertent intramuscular or subcutaneous injection have been reported. Symptoms may include vomiting, diarrhea, lethargy, inappetence, jaundice, and death associated with liver failure. Localized tissue necrosis at the injection site has also been reported. If inadvertent injection occurs, monitor the dog closely. Supportive therapy including IV fluids and treatment with gentamicin, tetracycline or trimethoprim/sulfa may be indicated. If anaphylactoid reaction occurs, use epinephrine.
2. Post vaccinal reactions consisting of mild canine cough syndrome may occur following use of this vaccine.
3. For veterinary use only. Store at 2° to 7°C (35° to 45°F). Use entire contents when first opened. Burn containers and all unused contents.
4. This vaccine contains penicillin, streptomycin and nystatin as preservatives.
Not For Parenteral Use
U.S. Veterinary License No. 165A
Schering-Plough Animal Health Corp., Omaha, Nebraska, 68103, USA
1 800 224-5318 (USA)
Copyright © 1999
Schering-Plough Animal Health Corp.
All rights reserved.
|
25x1 mL single dose vials with diluent & nasal applicators |
P13930-12, P13904-17 |
|
150x1 mL single dose vials with diluent & nasal applicators |
P13915-15, P13904-17 |
|
2x5 mL multiple dose vials with diluent & nasal applicators |
P13925-12 |
Nac No.
10471124556 MORRIS AVE., SUMMIT, NJ, 07901
| Telephone: | 862-245-4321 | |
| Order Desk: | 800-648-2118 | |
| Fax: | 862-245-4935 | |
| Customer Service: | 800-521-5767 | |
| Website: | www.intervet.com |
 |
Every effort has been made to ensure the accuracy of the Intra-Trac-II information published above. However, it remains the responsibility of the readers to familiarize themselves with the product information contained on the US product label or package insert. |
Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
