Excenel RTU EZ Sterile Suspension (Canada)
This page contains information on Excenel RTU EZ Sterile Suspension for veterinary use.The information provided typically includes the following:
- Excenel RTU EZ Sterile Suspension Indications
- Warnings and cautions for Excenel RTU EZ Sterile Suspension
- Direction and dosage information for Excenel RTU EZ Sterile Suspension
Excenel RTU EZ Sterile Suspension
This treatment applies to the following species: Company: Zoetis
Company: Zoetis
Ceftiofur Sterile Injectable Suspension
DIN 02317214
Veterinary Use Only
DESCRIPTION: EXCENEL® RTU EZ sterile suspension is a ready to use sterile suspension containing the hydrochloride salt of ceftiofur in a fractionated coconut oil vehicle.
Ceftiofur is a third generation cephalosporin antibiotic active against gram-positive and gram-negative bacteria including β-lactamase-producing strains. Like other cephalosporins, ceftiofur is bactericidal, in vitro, resulting from inhibition of cell wall synthesis.
Each mL of sterile suspension contains as medicinal ingredient, 50 mg ceftiofur (as ceftiofur hydrochloride), as non-medicinal ingredient Caprylic/Capric Triglyceride (fractionated coconut oil).
Figure 1. The chemical structure of ceftiofur hydrochloride
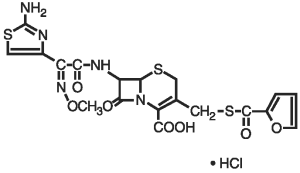
The chemical name of ceftiofur hydrochloride: 5-Thia-1-azabicyclo [4,2.0]oct-2-ene-2-carboxylic acid, 7-[[(2-amino-4-thiazolyl) (methoxyimino)-acetyl]amino]-3-[[(2-furanylcarbonyl) thio] methyl]-8-oxo-, hydrochloride salt [6R-[6a,7b(Z)]]-
Excenel RTU EZ Sterile Suspension Indications
Swine: EXCENEL RTU EZ sterile suspension is indicated for the treatment of swine bacterial respiratory disease (swine bacterial pneumonia) associated with Actinobacillus pleuropneumoniae and Pasteurella multocida.
Cattle (including lactating dairy cattle): EXCENEL RTU EZ sterile suspension is indicated for the treatment of:
● bovine respiratory disease (BRD, shipping fever, pneumonia) associated with Mannheimia haemolytica, Pasteurella multocida and Histophilus somni,
● acute bovine interdigital necrobacillosis (footrot, pododermatitis) associated with Fusobacterium necrophorum and Bacteroides melaninogenicus,
● acute post-partum metritis commonly associated with Arcanobacterium pyogenes (formerly Actinomyces pyogenes and prior to that Corynebacterium pyogenes), Escherichia coli and Fusobacterium necrophorum.
Dosage and Administration
Shake well before using (60 seconds or until the contents are completely re-suspended). The concentration of the suspension remains uniform for up to 25 minutes after thorough shaking. Injection in the neck region is recommended for both species to minimize edible tissue damage.
Proper injection technique should be used when administering EXCENEL RTU EZ sterile suspension including adjusting the needle insertion point to avoid major blood vessels and major nerves and using a needle of suitable gauge and length (16 gauge or larger, 1 to 1 1/2 inches long).
Swine: Administer by intramuscular (IM) injection only in swine 3.0 mg ceftiofur equivalents (CE) per kg of body weight (BW) (1 mL per 17 kg BW). Treatment should be repeated every 24 hours for a total of 3 treatments. Do not administer more than 5 mL per injection site.
Cattle: For bovine respiratory disease and acute bovine interdigital necrobacillosis: Administer by intramuscular or subcutaneous injection 1.0 mg ceftiofur equivalents (CE) per kg of body weight (BW) (1 mL per 50 kg BW). Treatment should be repeated every 24 hours for a total of 3 treatments. Additional treatments may be administered on days 4 and 5 for animals which do not show a satisfactory response (not recovered) after the initial 3 treatments. Do not administer more than 15 mL per injection site. For acute post-partum metritis: Administer by intramuscular or subcutaneous injection at a dosage of 2.2 mg ceftiofur per kg of body weight (2.2 mL per 50 kg body weight). Treatment should be repeated every 24 hours for a total of five treatments. Do not administer more than 15 mL per injection site.
Contraindications
The use of EXCENEL RTU EZ sterile suspension is contraindicated in animals previously found to be hypersensitive to the drug.
CAUTIONS: The use of ceftiofur in pregnant or lactating swine and in swine intended for breeding has not been evaluated and is therefore not recommended. Subcutaneous and intramuscular injection in cattle and intramuscular injection in swine can cause a transient local tissue reaction that may result in trim loss of edible tissue at slaughter.
Adverse Reactions
In safety studies, swelling at the injection site is commonly observed after subcutaneous administration to cattle and very rarely observed after intramuscular administration.Warnings
Treated animals must not be slaughtered for use as food for at least 2 days for swine and 3 days for cattle after the latest treatment with this drug. Do not use in veal calves. A withdrawal period has not been established in pre-ruminating calves. No milk withholding time is required when this product is used according to label directions and dosage. Use of dosages in excess of those indicated or administration by unapproved routes such as subcutaneous in swine and intramammary in cattle, may lead to illegal residues in edible tissues and/or in milk. To limit the development of antimicrobial resistance:
● EXCENEL RTU EZ sterile suspension should not be used as a mass medication for feedlot cattle and swine.
● EXCENEL RTU EZ sterile suspension should only be used to treat individual animals as per the Indications.
● The choice of EXCENEL RTU EZ sterile suspension as the most appropriate treatment should be confirmed by clinical experience supported where possible by pathogen culture and drug susceptibility testing.
● The extra-label drug use of EXCENEL RTU EZ sterile suspension is not recommended.
Penicillins and cephalosporins can cause allergic reactions in sensitized individuals. Topical exposures to such antimicrobials, including ceftiofur, may elicit mild to severe allergic reactions in some individuals. Repeated or prolonged exposure may lead to sensitization. Avoid direct contact of the product with the skin, eyes, mouth and clothing. Sensitization of the skin may be avoided by wearing latex gloves. Persons with a known hypersensitivity to penicillin or cephalosporins should avoid exposure to this product. In case of accidental eye exposure, flush with water for 15 minutes. In case of accidental skin exposure, wash with soap and water. Remove contaminated clothing. If allergic reaction occurs (e.g., skin rash, hives, difficult breathing), seek medical attention.
KEEP OUT OF REACH OF CHILDREN.
NOTE:
Swine: To reduce the possibility of excess trim at the injection site, do not slaughter for at least 14 days after the latest treatment with this drug.
Cattle: To reduce the possibility of excess trim at the injection site, do not slaughter for at least 28 days after the latest treatment with this drug.
Clinical Pharmacology
Swine: Ceftiofur administered as either ceftiofur sodium or ceftiofur hydrochloride is metabolized rapidly to desfuroylceftiofur, the primary metabolite. Administration of ceftiofur to swine as either the sodium or hydrochloride salt provides effective concentrations of ceftiofur and desfuroylceftiofur metabolites in plasma above the lowest minimum inhibitory concentration to encompass 90% of the most susceptible isolates (MIC90) for the labeled pathogens: Actinobacillus pleuropneumoniae and Pasteurella multocida for the 24 hour period between the dosing intervals.
Comparative Bioavailability Summary
EXCENEL RTU EZ Sterile Suspension is a lower-viscosity reformulation of EXCENEL RTU Sterile Suspension. Comparable plasma concentrations of ceftiofur administered as EXCENEL RTU Sterile Suspension or the reformulated EXCENEL RTU EZ Sterile Suspension were demonstrated in a comparative two-treatment, two-period crossover relative bioavailability study in swine. Products were administered via intramuscular (IM) injection into the neck, using alternating sides during periods 1 and 2. A summary of average plasma pharmacokinetic (PK) parameters in swine after a single IM administration of EXCENEL RTU Sterile Suspension and EXCENEL RTU EZ Sterile Suspension at a dose of 5.0 mg CE/kg BW is provided in Table 1.
Table 1: Comparative treatment values (arithmetic mean ± SD) for the plasma PK estimates of total ceftiofur (parent compound plus desfuroylceftiofur metabolites) in swine following an IM administration of 5.0 mg CE/kg BW, as either EXCENEL RTU (reference article) or as EXCENEL RTU EZ Sterile Suspension (test article).
|
PK Parameter |
EXCENEL RTU |
EXCENEL RTU EZ |
|
Cmax (µg/mL) |
18.2 ± 4.09 |
19.7 ± 3.39 |
|
AUC0-LOQ (µg*h/mL) |
257 ± 57.1 |
263 ± 54.8 |
|
tmax (h) |
1.5 ± 0.49 |
1.5 ± 0.73 |
|
t1/2 (h) |
20.0 ± 1.56 |
20.0 ± 1.82 |
|
t>0.2 (h) |
83.1 ± 10.3 |
82.5 ± 10.5 |
Cmax - maximum plasma concentration
AUC0-LOQ - the area under the plasma concentration vs. time curve from time of injection to the limit of quantification of the assay
tmax - the time after initial injection to when Cmax occurs
t1/2 - the plasma half life of the drug
t>0.2 - the time plasma concentrations remain above 0.2 µg/mL.
The standard bioequivalence (BE) criteria, based upon the exponentiated 90% confidence bounds about the ratio of treatment means, were met for the pivotal bioequivalence parameters, AUC0-LOQ and Cmax, when each formulation was administered to swine IM at a dose rate of 5.0 mg CE/kg BW (Table 2). Based on dose proportionality data, this finding of bioequivalence also applies to swine treated intramuscularly at the recommended label dose of 3.0 mg CE/kg BW.
Table 2: Back-transformed least squares (LS) means and 90% confidence interval (CI) for the two pivotal pharmacokinetic parameters, Cmax and AUC0-LOQ in swine following an IM administration of 5.0 mg CE/kg BW, as either EXCENEL RTU (reference article) or as EXCENEL RTU EZ Sterile Suspension (test article).
|
PK Parameter |
LS Mean Difference |
90% CI |
BE |
|
Cmax |
1.10 |
1.03 to 1.18 |
Yes |
|
AUC0-LOQ |
1.03 |
0.99 to 1.06 |
Yes |
If the 90% CI of the LS mean difference is within the limits of 0.80 to 1.25, then the results support bioequivalence of treatment groups.
In another comparative bioavailability study, comparable plasma concentrations of ceftiofur, administered as EXCENEL RTU Sterile Suspension or as EXCENEL Sterile Powder, were demonstrated when each product was administered intramuscularly at the label recommended dose of 3.0 mg CE/kg BW.
Cattle: Ceftiofur administered as either ceftiofur sodium or ceftiofur hydrochloride is metabolized rapidly to desfuroylceftiofur, the primary metabolite. Administration of ceftiofur to cattle as either the sodium or hydrochloride salt provides effective concentrations of ceftiofur and desfuroylceftiofur metabolites in plasma above the MIC90 for the label BRD pathogens Mannheimia haemolytica, Pasteurella multocida and Histophilus somni for at least 48 hours. The relationship between plasma concentrations of ceftiofur and desfuroylceftiofur metabolites above the MIC90 in plasma and efficacy has not been established for the treatment of bovine interdigital necrobacillosis (foot rot) associated with Fusobacterium necrophorum and Bacteroides melaninogenicus.
Comparative Bioavailability Summary
EXCENEL RTU EZ Sterile Suspension is a lower-viscosity reformulation of EXCENEL RTU Sterile Suspension. Comparable plasma concentrations of ceftiofur administered as EXCENEL RTU Sterile Suspension and the reformulated EXCENEL RTU EZ Sterile Suspension were demonstrated in two comparative two-treatment, two-period crossover relative bioavailability studies in cattle (one study comparing IM administration and one study comparing SC administration). A summary of average plasma pharmacokinetic (PK) parameters in cattle after a single IM or SC administration of EXCENEL RTU Sterile Suspension and EXCENEL RTU EZ Sterile Suspension at a dose of 2.2 mg CE/kg BW is provided in Table 3.
Table 3: Comparative treatment values (arithmetic mean ± SD) for the plasma PK estimates of total ceftiofur (parent compound plus desfuroylceftiofur metabolites) in cattle following an IM or SC administration of 2.2 mg CE/kg BW, as either EXCENEL RTU (reference article) or as EXCENEL RTU EZ Sterile Suspension (test article).
|
PK Parameter |
IM |
SC |
||
|
EXCENEL RTU |
EXCENEL RTU EZ |
EXCENEL RTU |
EXCENEL RTU EZ |
|
|
Cmax (µg/mL) |
8.58 ± 1.50 |
9.25 ± 1.73 |
8.40 ± 1.42 |
9.19 ± 1.65 |
|
AUC0-LOQ (µg*h/mL) |
89.4 ± 13.8 |
88.5 ±17.0 |
86.7 ± 20.3 |
91.0 ± 20.2 |
|
tmax (h) |
1.71 ± 0.706 |
1.73 ± 0.489 |
2.08 ± 0.670 |
2.25 ± 0.872 |
|
t1/2 (h) |
32.0 ± 8.48 |
29.3 ± 7.35 |
34.0 ± 8.52 |
32.9 ± 6.91 |
|
t>0.2 (h) |
42.2 ± 6.20 |
41.2 ± 6.11 |
40.5 ± 5.28 |
41.5 ± 7.32 |
Cmax - maximum plasma concentration
AUC0-LOQ - the area under the plasma concentration vs. time curve from time of injection to the limit of quantification of the assay
tmax - the time after initial injection to when Cmax occurs
t1/2 - the plasma half life of the drug
t>0.2 - the time plasma concentrations remain above 0.2 µg/mL
The standard bioequivalence (BE) criteria, based upon the exponentiated 90% confidence bounds about the ratio of treatment means, were met for the pivotal bioequivalence parameters, AUC0-LOQ and Cmax, when each formulation was administered to cattle IM or SC at a dose rate of 2.2 mg CE/kg BW (Table 4).
Table 4: Back-transformed least squares (LS) means and 90% confidence intervals (CI) for the two pivotal pharmacokinetic parameters, Cmax and AUC0-LOQ in cattle following an IM or SC administration of 2.2 mg CE/kg BW, as either EXCENEL RTU (reference article) or as EXCENEL RTU EZ Sterile Suspension (test article).
|
PK Parameter |
IM |
SC |
||
|
LS Mean Difference |
90% CI |
LS Mean Difference |
90% CI |
|
|
Cmax |
1.08 |
1.00 to 1.16 |
1.09 |
1.02 to 1.18 |
|
AUC0-LOQ |
0.984 |
0.94 to 1.03 |
1.06 |
0.99 to 1.13 |
In another comparative bioavailability study, comparable plasma concentrations of ceftiofur administered as EXCENEL RTU Sterile Suspension or as EXCENEL Sterile Powder were demonstrated when each product was administered intramuscularly or subcutaneously at the recommended dose of 1.0 mg CE/kg BW.
Microbiology
EXCENEL RTU EZ Sterile Suspension is a ready-to-use formulation that contains the hydrochloride salt of ceftiofur. Ceftiofur is a broad-spectrum cephalosporin antibiotic active against Gram-positive and Gram-negative bacteria. Like other cephalosporins, ceftiofur is predominantly bactericidal in vitro, resulting in the inhibition of cell wall synthesis. In vitro activity of ceftiofur has been demonstrated against Actinobacillus pleuropneumoniae and Pasteurella multocida, two pathogens associated with swine respiratory disease. Similarly, in vitro activity of ceftiofur has been demonstrated against Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni, the three major pathogens associated with bovine respiratory disease, and against Fusobacterium necrophorum and Bacteroides melaninogenicus, pathogenic anaerobic bacteria associated with bovine foot rot.
A summary of minimum inhibitory concentrations (MIC) is presented in Table 5 for swine. Isolates were obtained from field studies in the U.S. (1996/1997 and 2000/2001).
Table 5: Minimum Inhibitory Concentrations for Ceftiofur against Swine Clinical Isolates
|
Organism |
N |
MIC Range (µg/mL) |
MIC90 (µg/mL) |
|
Actinobacillus pleuropneumoniae |
28 |
0.03 - 0.06 |
0.03 |
|
Pasteurella multocida |
58 |
0.03 |
0.03 |
A summary of minimum inhibitory concentrations (MIC) is presented in Table 6 for Bovine. The bacterial isolates were obtained from clinical field studies in the USA collected in 1993.
Table 6: Minimum Inhibitory Concentrations for Ceftiofur against BRD Clinical Isolates.
|
Organism |
N |
MIC Range (µg/mL) |
MIC90 (µg/mL) |
|
Mannheimia haemolytica |
42 |
≤ 0.003-0.03 |
0.015 |
|
Pasteurella multocida |
48 |
≤ 0.003-0.015 |
≤ 0.003 |
|
Histophilus somni |
59 |
no range |
≤ 0.0019 |
Utilizing data that included isolates from swine and cattle affected by respiratory disease, zone diameter and minimum inhibitory concentration (MIC) breakpoints were determined using standardized procedures from the Clinical and Laboratory Standards Institute (CLSI) M31-A21 (currently Vet01-A4). The CLSI-accepted interpretive criteria for ceftiofur against these Gram-negative pathogens are shown in Table 7.
Table 7: CLSI-accepted interpretive criteria for ceftiofur against swine and cattle respiratory pathogens.*
|
Pathogen |
Disk Potency |
Zone diameter Interpretive standards (mm) |
MIC breakpoint (µg/mL) |
||||
|
S |
I |
R |
S |
I |
R |
||
|
Actinobacillus pleuropneumoniae, Pasteurella multocida |
30 µg |
≥21 |
18 to 20 |
≤17 |
≤2.0 |
4.0 |
≥8.0 |
|
Mannheimia haemolytica, Pasteurella multocida, Histophilus somni |
|||||||
|
S - Susceptible I - Intermediate R - Resistant |
|||||||
* These interpretive criteria are only intended for use when Vet01-A4 performance standards are used to determine antimicrobial susceptibility.
Efficacy
Swine: Plasma concentrations of ceftiofur administered as EXCENEL RTU Sterile Suspension or as EXCENEL RTU EZ Sterile Suspension following intramuscular administration in swine were compared and found to be bioequivalent for AUC0-LOQ and Cmax. Therefore, EXCENEL RTU EZ Sterile Suspension has the same efficacy profile as previously established for EXCENEL RTU Sterile Suspension. Because the efficacy of cephalosporin antibiotics is dependent upon time above MIC, EXCENEL RTU EZ Sterile Suspension is considered effective for the treatment of swine respiratory disease.
Cattle: Plasma concentrations of ceftiofur administered as EXCENEL RTU Sterile Suspension or as EXCENEL RTU EZ Sterile Suspension following intramuscular or subcutaneous administration in cattle were compared and found to be bioequivalent for AUC0-LOQ and Cmax. Therefore, EXCENEL RTU EZ Sterile Suspension is considered to have the same efficacy profile as previously established for EXCENEL RTU Sterile Suspension. Because the efficacy of cephalosporin antibiotics is dependent upon time above MIC, EXCENEL RTU EZ Sterile Suspension is considered effective for the labeled indications.
Animal Safety
Swine: Evaluation of target animal safety in swine was based on a PK comparison between the reformulated EXCENEL RTU EZ Sterile Suspension and EXCENEL RTU Sterile Suspension. Ceftiofur administered to swine as the reformulated EXCENEL RTU EZ Sterile Suspension at a dose of 5 mg CE/kg BW by IM injection was demonstrated to be bioequivalent to a corresponding IM injection of EXCENEL RTU Sterile Suspension based upon comparability of their respective AUC0-LOQ and Cmax values (see EFFICACY section). Because of the demonstrated blood level bioequivalence, this study confirms the systemic safety of the reformulated EXCENEL RTU EZ Sterile Suspension in swine when administered by IM injection at a dose of 5 mg CE/kg BW for three consecutive days.
Injection site tissue tolerance and resolution were evaluated after administering EXCENEL RTU EZ Sterile Suspension by intramuscular injection to 8 young pigs with the maximum proposed volume of 5 mL per injection site once daily for three consecutive days. Each injection was administered in a different location on the neck, and injection sites alternated between the left and right sides. General health and injection sites were evaluated through 42 days after the first treatment. No test article related health issues were observed. Mild swelling, erythema, and firmness were observed in a very small number of occasions (≤ 2% of total observations). No swelling was observed from 3 days after the last injection through the end of the study. Grossly visible discoloration of the injection site and histopathologic changes consistent with inflammation were noted in treated pigs necropsied 7 days or 14 days after injection.
Cattle: Evaluation of target animal safety in cattle was based on two PK studies comparing the reformulated EXCENEL RTU EZ Sterile Suspension and EXCENEL RTU Sterile Suspension (one study comparing IM administration and one study comparing SC administration). In both studies, ceftiofur, when administered to cattle at a dose of 2.2 mg CE/kg BW of the reformulated EXCENEL RTU EZ Sterile Suspension, was demonstrated to be bioequivalent to a 2.2 mg CE/kg BW dose of EXCENEL RTU Sterile Suspension (see EFFICACY section). Because of the demonstrated blood-level bioequivalence, these studies confirm systemic safety of the reformulated EXCENEL RTU EZ Sterile Suspension when administered either IM or SC at a dose of 2.2 mg CE/kg BW for five consecutive days.
Injection site tissue tolerance and lesion resolution were evaluated after administration of the reformulated EXCENEL RTU EZ Sterile Suspension by intramuscular and subcutaneous injections to 16 growing cattle (8 cattle for each route) at the maximum volume of 15 mL per injection site, once daily for five consecutive days. Each injection was administered in a different location on the neck and injection sites alternated between the left and right sides. General health and injection sites were evaluated through necropsy (up to 42 days after the first dose). Animals were euthanized on Day 7, 14, 28, or 42 (two calves at each time point). No test article-related health issues were observed. Injection site reactions consisted of firmness and swelling at the injection sites. Injection site swelling was observed in 4/1030 (0.4%) of IM injection site observations and in 606/1029 (58.9%) of SC injection site observations. Swelling progressively decreased over time, and was still present in both animals injected SC that were necropsied on Day 42. Grossly visible discoloration of the injection site and/or histopathologic changes consistent with inflammation were noted on Day 42 in SC (6 of 10) and (2 of 10) IM EXCENEL RTU EZ injection sites.
Storage
Store between 15 and 30°C. Protect from freezing. Contents should be used within 42 days after the first dose is removed.PRESENTATION: EXCENEL RTU EZ sterile suspension is available in 100 and 250 mL vials.
1 Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Approved Standard. CLSI Document M31-A2 (ISBN 1-56238-461-9). CLSI, 940 West Valley Road, Suite 1400, Wayne, Pennsylvania 19087-1898, 2002.
Zoetis is a trademark and Excenel is a registered trademark of Zoetis or its licensors, used under license by Zoetis Canada Inc.
Zoetis Canada Inc., Kirkland, QC, H9H 4M7
1740-11-2
40025957
CPN: 1198494.3
16,740 TRANS-CANADA HIGHWAY, KIRKLAND, QC, H9H 4M7
| Order Desk: | 800-663-8888 | |
| Technical Services Canada: | 800-461-0917 | |
| Technical Services USA: | 800-366-5288 | |
| Website: | www.zoetis.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |
Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
