The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
Excede Sterile Suspension for Horses
This page contains information on Excede Sterile Suspension for Horses for veterinary use.The information provided typically includes the following:
- Excede Sterile Suspension for Horses Indications
- Warnings and cautions for Excede Sterile Suspension for Horses
- Direction and dosage information for Excede Sterile Suspension for Horses
Excede Sterile Suspension for Horses
This treatment applies to the following species: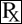 Company: Zoetis
Company: Zoetis
(Ceftiofur Crystalline Free Acid)
Sterile Suspension
For intramuscular injection in the horse.
Excede Sterile Suspension for Horses Caution
Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian. Federal law prohibits extra-label use of this drug in cattle for disease prevention purposes; at unapproved doses, frequencies, durations, or routes of administration; and in unapproved major food producing species/production classes.
Description
EXCEDE Sterile Suspension is a ready-to-use formulation that contains the crystalline free acid of ceftiofur, which is a broad spectrum cephalosporin antibiotic active against Gram-positive and Gram-negative bacteria including β-lactamase-producing strains. Like other cephalosporins, ceftiofur is bactericidal, in vitro, resulting from inhibition of cell wall synthesis.
Each mL of this ready-to-use sterile suspension contains ceftiofur crystalline free acid equivalent to 200 mg ceftiofur, in a caprylic/capric triglyceride and cottonseed oil based suspension.
Figure 1. Structure of ceftiofur crystalline free acid:
Chemical name of ceftiofur crystalline free acid:
7-[[2-(2-Amino-4-thiazolyl)-2-(methoxyimino)acetyl]amino]- 3-[[(2-furanylcarbonyl)thio] methyl]-8-oxo-5-thia-1- azabicyclo[4.2.0]oct-2-ene 2-carboxylic acid
Excede Sterile Suspension for Horses Indication
EXCEDE Sterile Suspension is indicated for the treatment of lower respiratory tract infections in horses caused by susceptible strains of Streptococcus equi ssp. zooepidemicus.
Excede Sterile Suspension for Horses Dosage And Administration
Shake well before using.
Administer two intramuscular injections to horses, 4 days apart, at a dose of 3.0 mg/lb (6.6 mg/kg). A maximum of 20 mL per injection site may be administered. Therapeutic drug concentrations are maintained for 6 days after the second injection (or a total of 10 days from the beginning of treatment) against Streptococcus equi ssp. zooepidemicus.
Table 1. Dosing Schedule for EXCEDE Sterile Suspension.
|
Weight (lb) |
Dose Volume (mL) |
|
100 |
1.5 |
|
200 |
3.0 |
|
300 |
4.5 |
|
400 |
6.0 |
|
500 |
7.5 |
|
600 |
9.0 |
|
700 |
10.5 |
|
800 |
12.0 |
|
900 |
13.5 |
|
1000 |
15.0 |
|
1100 |
16.5 |
|
1200 |
18.0 |
|
1300 |
19.5 |
|
1400 |
21.0 |
|
1500 |
22.5 |
|
1600 |
24.0 |
|
1700 |
25.5 |
|
1800 |
27.0 |
|
1900 |
28.5 |
|
2000 |
30.0 |
Contraindications
EXCEDE Sterile Suspension is contraindicated in horses with known allergy to ceftiofur or to β-lactam (penicillins and cephalosporins) group antimicrobials. Due to the extended exposure in horses, based on the drug’s pharmacokinetic properties, adverse reactions may require prolonged care.
Warnings
Not for use in humans. For use in animals only. Keep this and all drugs out of reach of children. Consult a physician in case of accidental human exposure.
Do not use in horses intended for human consumption.
Penicillins and cephalosporins can cause allergic reactions in sensitized individuals. Topical exposure to such antimicrobials, including ceftiofur, may elicit mild to severe allergic reactions in some individuals. Repeated or prolonged exposure may lead to sensitization. Avoid direct contact of the product with the skin, eyes, mouth and clothing. Sensitization of the skin may be avoided by wearing protective gloves. Persons with a known sensitivity to penicillin or cephalosporins should avoid exposure to this product. In the case of accidental eye exposure, flush with water for 15 minutes. In case of accidental skin exposure, wash with soap and water. Remove contaminated clothing. If allergic reaction occurs (e.g. skin rash, hives, difficult breathing) seek medical attention.
Antibacterial Warnings
Use of antibacterial drugs in the absence of a susceptible bacterial infection is unlikely to provide benefit to treated animals and may increase the risk of the development of drug-resistant bacteria.
Precautions
The administration of antimicrobials to horses under conditions of stress may be associated with acute diarrhea that can be fatal. If acute diarrhea is observed, additional doses of EXCEDE should not be administered and appropriate therapy should be initiated.
Due to the extended exposure in horses, based on the drug’s pharmacokinetic properties, adverse reactions may require prolonged care. EXCEDE is slowly eliminated from the body, with approximately 17 days needed to eliminate 97% of the dose from the body. Animals experiencing adverse reactions may need to be monitored for this duration of time.
The use of ceftiofur has not been evaluated in horses less than 4 months of age and in breeding, pregnant, or lactating horses. The long term effects on injection sites have not been evaluated.
Adverse Reactions
The injection of EXCEDE Sterile Suspension in the horse may cause firmness, swelling, sensitivity, and/or edema at the injection site (see ANIMAL SAFETY).
A total of 373 horses of various breeds, ranging in age from 4 months to 20 years, were included in the field study safety analysis. Adverse reactions reported in horses treated with EXCEDE and the placebo control are summarized in Table 2.
Injection site swelling (edema) was reported in 10 of 278 (3.6%) EXCEDE-treated horses and 1 of 95 (1%) of the placebo-treated horses. Of the 10 EXCEDE-treated horses with injection site swelling, 8 horses had swellings of 4 cm or less in diameter, one horse had a 10 cm diameter swelling and one horse had injection site reactions to both injections measuring 25 x 12 cm each. The injection site reactions in EXCEDE-treated horses resolved over 1 to 20 days.
At least one episode of diarrhea, loose, soft, or cowpie stools were observed in 25 of 278 (9%) of the EXCEDE-treated horses and 7 of 95 (7%) of the placebo-treated horses. The duration of episodes in EXCEDE-treated horses ranged from a single observation of loose stool to observations lasting 6 days. All cases were self-limiting and resolved with minimal (a single dose of loperamide) or no treatment.
Table 2. Number of Horses with Adverse Reactions During the Field Study with EXCEDE.
|
Adverse Reaction |
EXCEDE (n=278) |
Placebo (n=95) |
|
Diarrhea/Soft Stool |
25 (9%) |
7 (7%) |
|
Injection Site Swelling |
10 (4%) |
1 (1%) |
The material safety data sheet (MSDS) contains more detailed occupational safety information. To obtain a material safety data sheet or to report any adverse event please call 1-888-963-8471.
Clinical Pharmacology
Ceftiofur is a beta-lactam antibiotic from the cephalosporin class. Beta lactams exert their inhibitory effect by interfering with bacterial cell wall synthesis. This interference is primarily due to its covalent binding to the penicillin-binding proteins, which are essential for synthesis of the bacterial wall. Ceftiofur administered as either ceftiofur sodium (NAXCEL® Sterile Powder) or ceftiofur crystalline free acid (EXCEDE Sterile Suspension) is rapidly metabolized to desfuroylceftiofur, the primary metabolite with antimicrobial activity. Two intramuscular injections of EXCEDE Sterile Suspension at a dose of 6.6 mg/kg body weight in the horse provide concentrations of ceftiofur and desfuroylceftiofur related metabolites in plasma above the therapeutic target of 0.2 µg/mL for the entire 96 hour (4 day) dosing interval and for 6 days after the second injection (or a total of 10 days from the beginning of treatment) (see Figure 2 and Table 3).
Figure 2. Average plasma concentration of ceftiofur and desfuroylceftiofur related metabolites in horses following the intramuscular administration of either EXCEDE Sterile Suspension at a dose of 3.0 mg/lb (6.6 mg/kg) administered twice at a 96 hour interval or NAXCEL Sterile Powder at a dose of 1.0 mg/lb (2.2 mg/kg BW) once daily for 10 consecutive days.
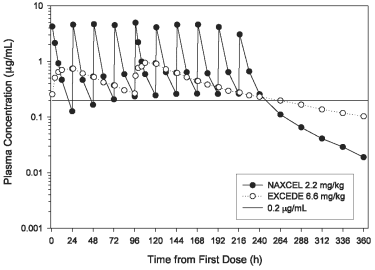
Table 3. Pharmacokinetic parameters measured after either two intramuscular injections of EXCEDE Sterile Suspension at a dose of 3.0 mg/lb (6.6 mg/kg) BW at a 96 hour interval or NAXCEL Sterile Powder at a dose of 1.0 mg/lb (2.2 mg/kg) BW once daily for 10 consecutive days are summarized in the following table.
|
PK Parameter |
CCFA-SS at 6.6 mg/kg BW administered twice 96 h apart (Mean ± SD; n=12) |
Ceftiofur sodium at 2.2 mg/kg BW once daily for 10 days (Mean ± SD; n=11) |
||
|
AUC 0-∞ (µg•h/mL) |
157 (19.1) |
353 (44.9) |
||
|
t>0.2 (h) |
262 (29.0) |
ND |
||
|
|
Dose 1 |
Dose 2 |
Dose 1 |
Dose 10 |
|
Tmax (h) |
21.6 (5.8) |
15.6 (6.3) |
1.0 |
2.0 (3.3) |
|
Cmax (µg/mL) |
0.78 (0.19) |
1.0 (0.24) |
4.31 ± 0.78 |
3.99 (1.23) |
Microbiology
Ceftiofur is a cephalosporin antibiotic. Like other ß-lactam antimicrobials, ceftiofur exerts its inhibitory effect by interfering with bacterial cell wall synthesis. This interference is primarily due to its covalent binding to the penicillin-binding proteins (PBPs) (i.e., transpeptidase and carboxypeptidase), which are essential for synthesis of the bacterial wall. Ceftiofur is not active against Pseudomonas spp. and enterococci.
The minimum inhibitory concentration (MIC) values for ceftiofur against label-claim pathogens isolated from lower respiratory tract infections in horses enrolled in a 2007-2008 field effectiveness study are presented in Table 4. All MICs were determined in accordance with the Clinical and Laboratory Standards Institute (CLSI) standards.
Table 4. Activity of EXCEDE Against Pathogens Isolated from Horses Treated With EXCEDE in Field Studies in the U.S. During 2007-2008.
|
Disease |
Pathogen |
Treatment Outcome |
# of Isolates |
Time of Sample Collection |
MIC50 µg/mL |
MIC90 µg/mL |
MIC Range µg/mL |
|
Lower Respiratory Tract Infection |
Streptococcus equi ssp. zooepidemicus |
Success |
93* |
Pre-Treatment |
0.06 |
0.12 |
0.03-0.5 |
|
Failure |
42 |
Pre-Treatment |
0.06 |
0.25 |
0.03-0.5 |
* One horse cultured Staphylococcus aureus (successfully treated) and is not represented in the table.
Effectiveness
A double masked, randomized, negative control, field study evaluated the effectiveness of two intramuscular doses of 6.6 mg/kg EXCEDE Sterile Suspension administered 4 days apart for the treatment of lower respiratory infections caused by Streptococcus equi ssp. zooepidemicus in the horse. In this study, a total of 278 horses were treated with EXCEDE, and 95 horses were treated with saline injections. One hundred ninety-three horses (136 EXCEDE and 57 saline placebo) were included in the statistical analysis. Therapeutic success was characterized by no worsening of clinical signs at Day 4, clinical improvement at Day 9, resolution of the clinical signs by Day 15, and no recurrence of clinical signs by Day 25 after initial dosing. EXCEDE was superior to the saline control. Table 5 summarizes the clinical success rates obtained 15 and 25 days after the first dose.
Table 5. Clinical success rates at Day 15 and 25.
|
Effectiveness parameter |
EXCEDE |
Saline Control |
P-value |
|
Clinical success Day 15 |
73.53% |
38.60% |
N/A |
|
Clinical success Day 25 |
69.12% |
31.58% |
0.0215 |
Animal Safety
Two studies, a target animal safety (TAS) study and a pharmacokinetic (PK) study (see CLINICAL PHARMACOLOGY section), were conducted to assess the safety of EXCEDE in the horse.
In the TAS study, healthy adult horses received 6 intramuscular (lateral neck) injections of EXCEDE Sterile Suspension at doses of either 3.0 (1X), 6.0 (2X) or 9.0 (3X) mg/lb with a 4 day interval between each injection. In the TAS study, there were no treatment related gastrointestinal findings for the three EXCEDE Sterile Suspension treatment groups. In the PK study, one horse treated with 6.0 mg/lb (2X) EXCEDE experienced a mild episode of colic the day after the second injection of EXCEDE. The horse recovered without treatment.
Injection sites were observed in both studies. In both studies, the largest injection volume administered was 20 mL per injection site. There were no observations of erythema, necrosis or drainage at the injection sites in these studies. Firmness, swelling, and/or sensitivity were observed in at least one injection site in all horses treated at the label dose. In the TAS study, injection site reaction measurements ranged from no measurable reaction to 16 x 33 x 1.5 cm. In the PK study, the largest area of edema associated with the injection site ranged from no detectable reaction to a 30 x 36 cm area of edema. Injection site reactions developed within 2 days of injection and resolved within 1-18 days. In the PK study, 2 horses had small areas of firmness that had not resolved at the end of the study (21 days after injection). In both studies, a greater incidence of injection site reactions occurred after the second injection, and in several horses, swelling at the injection site resolved then recurred 1-5 days later.
In the PK study, several horses developed clinical signs consistent with foot pain (stiff in the front limbs when turned in tight circles, and increased pulses and heat to the front feet). One horse in the NAXCEL group and one horse in the 6.0 mg/lb (2X) EXCEDE group were euthanized due to laminitis. Clinical signs of foot pain (stiff front limbs and increased heat and pulses in feet) affected more horses, for a longer period of time, in all EXCEDE-treated groups as compared to the NAXCEL-treated group. The study housing (multi-horse pens on concrete slabs) and diet (free choice alfalfa/grass mix and once a day pellets) may have contributed to the development of foot pain. The prevalence and severity of injection site reactions in EXCEDE-treated horses may also have contributed to the development of a stiff gait. A causal relationship between ceftiofur and foot pain could not be definitively determined.
Storage Conditions
Store at controlled room temperature 20° to 25°C (68° to 77°F). Shake well before using. Contents should be used within 12 weeks after the first dose is removed.
How Supplied
EXCEDE Sterile Suspension is available in the following package sizes:
100 mL vial
250 mL vial
Approved by FDA under NADA # 141-209
Distributed by: Zoetis Inc., Kalamazoo, MI 49007
www.EXCEDE.com or call 1-888-963-8471
Revised: November 2018
40025091
CPN: 3690486.1
333 PORTAGE STREET, KALAMAZOO, MI, 49007
| Telephone: | 269-359-4414 | |
| Customer Service: | 888-963-8471 | |
| Website: | www.zoetis.com |
 |
Every effort has been made to ensure the accuracy of the Excede Sterile Suspension for Horses information published above. However, it remains the responsibility of the readers to familiarize themselves with the product information contained on the US product label or package insert. |
Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
