Equicaine (Canada)
This page contains information on Equicaine for veterinary use.The information provided typically includes the following:
- Equicaine Indications
- Warnings and cautions for Equicaine
- Direction and dosage information for Equicaine
Equicaine
This treatment applies to the following species:mepivacaine hydrochloride injection, USP
DIN 01910043
VETERINARY USE ONLY
THERAPEUTIC OR PHARMACOLOGICAL CLASSIFICATION
Local anaesthetic with rapid and prolonged effect for use in horses and dogs
sterile
Chemistry
Mepivacaine Hydrochloride:
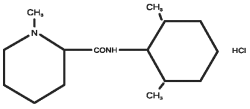
Chemical Name:
1-methyl-2, 6-pipecoloxylidide monohydrochloride
Description
Mepivacaine hydrochloride is a white, crystalline, odourless powder, readily soluble in water, and very stable in aqueous solution. Solution can be boiled for several hours, autoclaved repeatedly, if desired, or stored for extended periods of time without risk of decomposition.
Medicinal Ingredient:
|
mepivacaine hydrochloride |
20 mg/mL |
Non-medicinal ingredients:
|
methylparaben |
1 mg/mL |
|
sodium chloride |
to make isotonic in water for injection |
Action
Mepivacaine hydrochloride is a local anaesthetic which produces rapid and marked anaesthesia lasting for several hours. Its pharmacological properties are somewhat similar to those of lidocaine, which it resembles chemically. Its action, however, is more rapid in onset and somewhat more prolonged than that of lidocaine. This rapid onset and long duration of anaesthesia enables the practitioner to proceed with intended manipulations without delay and to complete work under desensitization which is adequate even for prolonged operations. This activity may be enhanced by the addition of epinephrine 1:100,000. The addition should be carried out aseptically for current use and any unused portion should be discarded. The compound has shown excellent tissue compatibility in laboratory animals and in clinical use.
Equicaine Indications
EQUICAINE® (mepivacaine hydrochloride) is recommended for infiltration, nerve block, intraarticular and epidural anaesthesia for horses and dogs. It has also been found useful for topical anaesthesia of the laryngeal mucosa prior to ventriculectomy. As with other local anaesthetics, the dosage varies considerably depending on the anaesthetic technique, body area to be desensitized and the surgical procedure. Pharmacological studies in various species of animals have shown that the drug produces complete and effective anaesthesia at dosages that are no more than half those needed when procaine is used.
Equicaine Dosage And Administration
The following dosages have generally proved satisfactory and are therefore suggested as a guide:
For nerve block:
(diagnosis of lameness, dental procedures, pain relief in osteoarthritis, navicular disease)-
HORSE: 3 to 15 mL
DOG: 2 to 5 mL
For epidural anaesthesia:
HORSE: 5 to 20 mL
DOG: 2 to 10 mL
For intraarticular anaesthesia:
(removal of fracture chips, bone and bog spavin, arthritis)
HORSE: 10 to 15 mL
For infiltration:
HORSE: up to 200 mL for a 500 kg horse
DOG: up to 20 mL for a 50 kg dog
For anaesthesia of the laryngeal mucosa prior to ventriculectomy:
HORSE: EQUICAINE™ may be administered topically or by infiltration or by a combination of the two.
For topical administration, a total of 25 to 40 mL applied by spray (3 mL/application) is usually adequate.
For infiltration, 20 to 50 mL will suffice.
Contraindications
Use of EQUICAINE® (mepivacaine hydrochloride) is contraindicated when:
(i) animals are allergic to mepivacaine, and
(ii) when area to be infiltrated is infected, fibrosed or deficient in circulation.
Warnings
This drug is not to be administered to horses that are to be slaughtered for use in food.
Precautions
When administered by skilled persons, EQUICAINE® (mepivacaine hydrochloride) may be employed safely for local infiltration, for common nerve blocking procedures, for intraarticular and epidural anaesthesia. The following precautions, which are observed with respect to all local anaesthetics, also apply to this anaesthetic:
(1) Injections should always be made aseptically and with frequent aspirations. If blood is aspirated, the needle should be relocated and the injections continued cautiously.
(2) When used for epidural anaesthesia, care should be taken to avoid injection into the subarachnoid space. The skin should be shaved and sterilized, and the needles used must be sharp and of proper length.
(3) The depth of anaesthesia should be checked by pricking the area before manipulations are begun.
(4) When used with epinephrine precautions required for any vasopressor drug should be observed.
Safety And Tolerance
Clinical investigations in animals demonstrated that EQUICAINE® (mepivacaine hydrochloride) (for local anaesthesia) has a low level of toxicity. It is well tolerated and complications have not been observed when the drug was used in the recommended doses.
Storage
Store between 15 and 30°C.
Presentation
EQUICAINE® (mepivacaine hydrochloride injection USP) is supplied in rubber-capped, multiple dose vials of 50 mL.
Zoetis is a trademark and Equicaine is a registered trademark of Zoetis or its licensors, used under license by Zoetis Canada Inc.
Zoetis Canada Inc., Kirkland QC H9H 4M7
1693-11-2
4025107 - Ph 1295
CPN: 1198456.2
16,740 TRANS-CANADA HIGHWAY, KIRKLAND, QC, H9H 4M7
| Order Desk: | 800-663-8888 | |
| Technical Services Canada: | 800-461-0917 | |
| Technical Services USA: | 800-366-5288 | |
| Website: | www.zoetis.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |
Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
