Droncit Injectable Cestocide (Canada)
This page contains information on Droncit Injectable Cestocide for veterinary use.The information provided typically includes the following:
- Droncit Injectable Cestocide Indications
- Warnings and cautions for Droncit Injectable Cestocide
- Direction and dosage information for Droncit Injectable Cestocide
Droncit Injectable Cestocide
This treatment applies to the following species: Company: Elanco
Company: Elanco
(praziquantel)*
56.8 mg/mL Sterile Solution
Injectable Cestocide For Dogs and Cats
VETERINARY USE ONLY
*2-(cyclohexylcarbonyl)-1,2,3,6,7,11 b-hexahydro-4H-pyrazino[2, 1-a]isoquinolin-4-one
DIN 02169509
Description
DRONCIT Injectable Cestocide is a clear solution containing 56.8 milligrams of praziquantel per mL which has been formulated for subcutaneous or intramuscular use in dogs and cats.
Droncit Injectable Cestocide Indications
DRONCIT (praziquantel) Injectable Cestocide is indicated for the removal of the following cestodes Canine: Dipylidium caninum, Taenia pisiformis, Taenia hydatigena, Echinococcus granulosus, Mesocestoides corti and for the removal and control of Echinococcus multilocularis. Feline: Taenia taeniaeformis and Dipylidium caninum.
ACTION: DRONCIT (praziquantel) is absorbed, metabolized in the liver and excreted in the bile. Upon entering the digestive tract from the bile, cestocidal activity is exhibited.(1) Following exposure to praziquantel, the tapeworm loses its ability to resist digestion by the mammalian host. Because of this, whole tapeworms including the scolex, are very rarely passed after administration of praziquantel. In many instances only disintegrated and partially digested pieces of tapeworms will be seen in the stool. The majority of tapeworms killed are digested and are not found in the feces.
USE DIRECTIONS: DRONCIT (praziquantel) Injectable Cestocide may be administered by either the subcutaneous or intramuscular route to both dogs and cats. The minimum effective dosage of praziquantel varies according to body weight. Smaller animals require relatively larger doses because of their higher metabolic rate. The optimum dose for each individual animal will be achieved by utilizing the following dosage schedule.
DOGS
|
2.3 kg (5 lb) and under |
0.3 mL |
|
2.7 - 4.5 kg (6-10 lb) |
0.5 mL |
|
5 - 11.4 kg (11-25 lb) |
1.0 mL |
|
Over 11.4 kg (25 lb) |
0.2 mL/2.3 kg (5 lb) (To a maximum of 3.0 mL) |
CATS
|
2.3 kg (5 lb) and under |
0.2 mL |
|
2.7 - 4.5 kg (6-10 lb) |
0.4 mL |
|
5 kg (11 lb) and over |
0.6 mL maximum |
Not intended for use in kittens and puppies less than four weeks of age.
FOR USE ONLY UNDER THE DIRECTION OF A VETERINARIAN.
FASTING: The recommended dosage of praziquantel is not affected by the presence or absence of food in the gastrointestinal tract, therefore, FASTING IS NEITHER NECESSARY NOR RECOMMENDED.
MEDICINAL INGREDIENT PER mL: 56.8 mg praziquantel
NON-MEDICINAL INGREDIENTS PER mL: 75 mg benzyl alcohol
5 mg chlorobutanol hydrous
propylene glycol q.s.
ADMINISTRATION: DRONCIT (praziquantel) Injectable Cestocide may be administered by either the subcutaneous or intramuscular route to both dogs and cats. The intramuscular route may be preferred in dogs due to brief periods of pain that infrequently follow subcutaneous administration.
RETREATMENT: For those animals living where reinfections are likely to occur, clients should be instructed in the steps to optimize prevention, otherwise retreatment may be necessary. This is true in cases of Dipylidium caninum where reinfection is almost certain to occur if fleas are not removed from the animal and its environment. In addition, for control of Echinococcus multilocularis, a program of regular treatment every 21 to 26 days may be indicated.
Echinococcus multilocularis is a tapeworm species ordinarily considered to be found in wild canids, including foxes, coyotes and wolves. The parasite has also been identified in domestic dogs and cats and potentially is a serious public health concern by involving humans as accidental intermediate hosts.
The life cycle of the parasite is based on a predator-prey relationship, as depicted below.
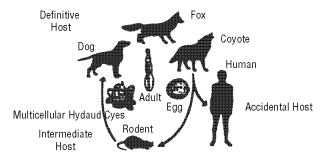
The adult tapeworm is small (1-4 mm) and resides in the intestinal tract of the definitive host (wild or domestic canids). Eggs from the adult tapeworm are shed in the feces of the infected canid. Rodents such as mice and voles serve as the intermediate host for E. multilocularis. Eggs ingested by rodents develop in the liver, lungs and other organs to form multilocular cysts.
The life cycle is completed after a canid consumes a rodent infected with cysts. After ingestion of an infected rodent, larvae within the cyst develop to adult tapeworms in the intestinal tract of the canid. Eggs may begin to be passed in the feces of the canid approximately 28 days later.
This parasite poses a serious public health problem because of the possibility for human involvement in the life cycle. If eggs shed by an infected canid are accidentally ingested, a highly pathogenic condition (Alveolar Hydatid Disease) results from development of the cyst stage in humans.
The original geographic distribution of E. multilocularis was primarily confined to northern areas of North America. Current evidence indicates migration of the parasite well into the continental United States.2 3
Domestic dogs living in E. multilocularis endemic areas that roam freely with the opportunity to catch wild rodents, are at risk for infection. Pet owners should be advised on how to minimize this risk. Proper restraint of roaming dogs should be encouraged, along with regular treatment with DRONCIT, following the dosing schedule and precautions indicated below.
Additional information on the life cycle and epidemiology of this parasite is available in veterinary parasitology texts.4 5
DIAGNOSIS: Diagnosis of E. multilocularis in canids is difficult. The adult tapeworm produces no clinical signs of infection. Tapeworm segments (proglottids) are usually not observed in the feces. E. multilocularis eggs, observed using microscopic fecal examination procedures, are similar in appearance to the common taenid species of canids such as Taenia pisiformis.
Assistance in the diagnosis of E. multilocularis may be available from a provincial veterinary diagnostic laboratory. Additional information regarding areas where E. multilocularis is suspected or has been confirmed may be obtained from area veterinary schools.
TREATMENT: Dogs infected with E. multilocularis should be treated to prevent exposure of humans to infective eggs and to reduce perpetuation of the parasite’s life cycle.
The dosage of DRONCIT for removal of E. multilocularis is the same as that indicated for the removal of the other tapeworm species listed on the label. Laboratory efficacy studies have demonstrated the recommended dosage is 100% effective for removal of this tapeworm. Under condition of continual exposure to wild rodents, retreatment of the dog at 21-26 day intervals is recommended to prevent the shedding of infectious eggs.
Precautions
Although not observed in clinical studies, an anaphylactoid reaction can occur with any drug and should be treated symptomatically, if it occurs. Strict hygienic precautions should be taken when handling dogs or feces suspected of harbouring E. multilocularis. Infected dogs treated for the first time with DRONCIT and dogs treated at intervals greater than 28 days may shed eggs in the feces after treatment. The animal should be held in the clinic during this interval and all feces should be incinerated or autoclaved. If these procedures are not possible, the eggs can be destroyed by soaking the feces in a sodium hypochlorite (bleach) solution of 3.75% or greater.6 All areas where the animal was maintained or in contact with should be thoroughly cleaned with sodium hypochlorite and allowed to dry completely before re-use.OVERDOSAGE: Dosages of 5 times the labelled rate administered to cats and dogs did not produce signs of clinical toxicity following either intramuscular or subcutaneous injections of the formulation. Symptoms of overdosage include vomiting, excessive salivation and mild depression.
Warning
Keep out of the reach of children. Not for human use.Storage
Store below 25°C.How Supplied
10 mL vials.References
1 Pharmacokinetic Studies with DRONCIT in Animals Using a Biological Assay. P. Andrews, Veterinary Medicine Review, 2/76, pg. 154-165. DRONCIT is a Reg. TM of the parent company of Farbenfabriken Bayer Gmbh, Leverkusen.
2 Hildreth, M.B., Johnston, M.D. and Kazacos K.R., 1991. A Zoonosis of Increasing Concern in the United States. Compendium for Continuing Education, 13(5) 727-740.
3 Lieby, P.D., Carney, W.P., and Woods, C.E., 1970. Studies on Sylvatic Echinococcus multilocularis in the North Central United States. J. Parasite 56 (6) 1141-1150.
4 Georgi, J.R. and Georgi M.E., 1990. Parasitology for Veterinarians, W.B. Saunders Co. 118-138.
5 Soulsby, E.J.L., 1982. Helminths, Arthropods and Protozoa of Domesticated Animals. 7th Edition. Lea and Febigir. 118-138.
6 Craig, P.S. and McPharson C.N.L., 1988 Sodium Hypochlorite as an Ovicide for Echinococcus. Ann Trop Med and Parasite 82 (2) 211-213.
Elanco Canada Limited, 1919 Minnesota Court, Suite 401, Mississauga, Ontario L5N 0C9
1-800-265-5475
Droncit, Elanco and the diagonal bar logo are trademarks of Elanco or its affiliates.
© 2022 Elanco or its affiliates.
26Sep2022
CPN: 1231212.1
1919 MINNESOTA COURT, SUITE 401, MISSISSAUGA, ON, L5N 0C9
| Customer Service: | 800-265-5475 | |
| Fax: | 519-821-7831 | |
| Website: | www.elanco.ca | |
| Email: | elancocanadacustomerservice@elancoah.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |
Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
