Vontrol Prescribing Information
Package insert / product label
Generic name: diphenidol
Dosage form: Tablets
Medically reviewed by Drugs.com. Last updated on Mar 25, 2024.
On This Page
‘Vontrol’ may cause hallucinations, disorientation, or confusion. For this reason, its use is limited to patients who are hospitalized or under comparable, continuous, close, professional supervision. Even then, the physician should carefully weigh the benefits against the possible risks and give due consideration to alternate therapeutic measures.
Vontrol Description
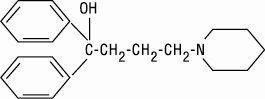
Diphenidol, α, α-diphenyl-1-piperidinebutanol, is a compound not related to the antihistamines, phenothiazines, barbiturates, or other agents with antivertigo or antiemetic action. It has this configuration:
Each round, orange ‘Vontrol’ tablet is debossed SKF and 25 and contains diphenidol hydrochloride equivalent to diphenidol, 25 mg. Inactive ingredients consist of acacia, calcium sulfate, cellulose, FD&C Yellow No. 5 (tartrazine), FD&C Yellow No. 6, magnesium stearate and starch.
Vontrol - Clinical Pharmacology
‘Vontrol’ (diphenidol, SK&F) apparently exerts a specific antivertigo effect on the vestibular apparatus to control vertigo and inhibits the chemoreceptor trigger zone to control nausea and vomiting.
Indications and Usage for Vontrol
- VERTIGO—‘Vontrol’ is indicated in peripheral (labyrinthine) vertigo and associated nausea and vomiting, as seen in such conditions as: Meniere’s disease, middle- and inner-ear surgery (labyrinthitis).
- NAUSEA AND VOMITING—‘Vontrol’ is indicated in the control of nausea and vomiting, as seen in such conditions as: postoperative states, malignant neoplasms and labyrinthine disturbances.
Contraindications
Known hypersensitivity to the drug is a contraindication. Anuria is a contraindication. (Since approximately 90% of the drug is excreted in the urine, renal shutdown could cause systemic accumulation.)
Warnings
‘Vontrol’ (diphenidol, SK&F) may cause hallucinations, disorientation or confusion. For this reason, its use is limited to patients who are hospitalized or under comparable, continuous, close, professional supervision. Even then, the physician should carefully weigh the benefits against the possible risks and give due consideration to alternate therapeutic measures.
The incidence of auditory and visual hallucinations, disorientation and confusion appears to be less than ½% or approximately one in 350 patients. The reaction has usually occurred within three days of starting the drug in recommended dosage and has subsided spontaneously usually within three days after discontinuation of the drug. Patients on ‘Vontrol’ should be observed closely and in the event of such a reaction the drug should be stopped.
Usage in Pregnancy
Use of any drug in pregnancy, lactation or in women of childbearing age requires that the potential benefits of the drug be weighed against its possible hazards to the mother and child.
In animal teratogenesis and reproduction studies of ‘Vontrol’ (diphenidol, SK&F), there were no significant differences between drug-treated groups and untreated control groups, except as noted under animal Reproduction Studies (see “Pharmacology [animal],” column 4).
In 936 patients who received ‘Vontrol’ during pregnancy, the incidences of normal and abnormal birth were comparable to those reported in the literature for the average population of pregnant patients. And in no instance was there any evidence that ‘Vontrol’ played a part in birth abnormality (see “In Pregnancy,” column 5).
‘Vontrol’ is not indicated for use in nausea and vomiting of pregnancy, since the therapeutic value and safety in this indication have not yet been determined.
Precautions
The antiemetic action of ‘Vontrol’ (diphenidol, SK&F) may mask signs of overdose of drugs (e.g., digitalis) or may obscure diagnosis of conditions such as intestinal obstruction and brain tumor.
Although there have been no reports of blood dyscrasias with ‘Vontrol’, patients should be observed regularly for any idiosyncratic reactions.
‘Vontrol’ has a weak peripheral anticholinergic effect and should be used with care in patients with glaucoma, obstructive lesions of the gastrointestinal and genitourinary tracts, such as stenosing peptic ulcer, prostatic hypertrophy, pyloric and duodenal obstruction, and organic cardiospasm.
‘Vontrol’ Tablets contain FD&C Yellow No. 5 (tartrazine) which may cause allergic-type reactions (including bronchial asthma) in certain susceptible individuals. Although the overall incidence of FD&C Yellow No. 5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity.
Adverse Reactions/Side Effects
Auditory and visual hallucinations, disorientation and confusion have been reported. Drowsiness, overstimulation, depression, sleep disturbance, dry mouth, g.i. irritation (nausea and indigestion), or blurred vision may occur.
Rarely, slight dizziness, skin rash, malaise, headache, or heartburn may occur. Mild jaundice of questionable relationship to the use of ‘Vontrol’ (diphenidol, SK&F) has been reported. Slight, transient lowering of blood pressure has been reported in a few patients.
(See laboratory studies under “Pharmacology [human],” Column 4.)
ADULTS—FOR VERTIGO OR NAUSEA AND VOMITING
The usual dose is one tablet (25 mg) every four hours as needed. Some patients may require two tablets (50 mg).
CHILDREN—FOR NAUSEA AND VOMITING
These recommendations are for nausea and vomiting only. There has been no experience with ‘Vontrol’ in vertigo in children.
Unit doses in children are best calculated by body weight: usually 0.4 mg./lb.
Children’s doses usually should not be given more often than every four hours. However, if symptoms persist after the first dose, administration may be repeated after one hour. Thereafter, doses may be given every four hours as needed.
The total dose in 24 hours should not exceed 2.5 mg./lb.
NOTE: The drug is not recommended for use in children under 50 pounds. The dosage for children 50 to 100 pounds is one tablet (25 mg.).
Overdosage
In the event of overdosage, the patient should be managed according to his symptoms. Treatment is essentially supportive, with maintenance of blood pressure and respiration, plus careful observation. Early gastric lavage may be indicated depending on the amount of overdose and nature of symptoms.
How is Vontrol supplied
Tablets containing 25 mg. diphenidol, as the hydrochloride, in bottles of 100.
PHARMACOLOGY (ANIMAL)
‘Vontrol’ (diphenidol, SK&F) exerts its antiemetic effect primarily by inhibiting the chemoreceptor trigger zone, as evidenced by its activity in blocking emesis induced by apomorphine in dogs. In this regard ‘Vontrol’, as the hydrochloride salt, has a potency equal to the potent phenothiazine antiemetic, chlorpromazine hydrochloride. In animals ‘Vontrol’ has only weak parasympatholytic activity and no significant sedative, tranquilizing or antihistaminic action or effects on blood pressure, heart rate, respiration or the electrocardiogram.
Subacute and chronic toxicity studies in rats and dogs, in which large doses of ‘Vontrol’, as the hydrochloride salt, were administered orally and intramuscularly for periods up to one year, revealed no significant effects on hematology, liver function, kidney function or blood glucose determinations. Histological examination of the animals’ tissues did not reveal any significant lesions attributable to administration of ‘Vontrol’.
Reproduction Studies
Teratogenesis and reproduction studies were carried out in rats and rabbits. In rats, ‘Vontrol’ (diphenidol, SK&F), as the hydrochloride salt, was fed daily to male and female animals in doses of 20 mg./kg. and 40 mg./kg. (approximately three and six times the maximum recommended daily dose in adult humans) for 60 days before mating, and during mating, gestation and lactation for each of two litters. There were no significant differences between drug-treated and untreated control groups with regard to conception rate, litter size, live birth or viability in either of the two litters. There was no congenital anomaly among the offspring. In rabbits, ‘Vontrol’, as the hydrochloride salt, was fed in the diets in doses of 5 mg./kg. or 75 mg./kg. (approximately equal to, and 12 times as much as, the maximum recommended daily dose in adult humans) from the first day of gestation through the 26th or 27th day of gestation, when the young were delivered by Cesarean section. There were no significant differences between drug-treated and control groups with regard to number and weight of fetuses, numbers of resorption sites or viable fetuses. There was also no statistically significant difference between drug-treated and control groups with regard to the total percentage of underdeveloped fetuses. However, when data were calculated on the basis of a ratio between underdeveloped fetuses and number of pregnant does, an adverse dose-related effect was observed in the high-dose test group.
PHARMACOLOGY (HUMAN)
Three double-blind controlled studies comparing ‘Vontrol’ (diphenidol, SK&F) to placebo were carried out: one in 32 male volunteers over a four-week period; one in 45 volunteers of whom 15 were studied for 12 weeks and 17 for 24 weeks; and one in 48 volunteers of whom 36 were studied for 12 weeks.
In the first study ‘Vontrol’, as the hydrochloride salt, was given orally in daily doses that were started at 75 mg. during the first week and graduated up to 200 mg. by the fourth week. In the second study, one group received ‘Vontrol’ orally, as the hydrochloride salt, titrated up to 500 mg. daily, then down to 200 mg. daily; another group received a maximum of 200 mg. daily. In the third study, patients received oral doses of 200 mg. to 300 mg. of ‘Vontrol’ daily, as the hydrochloride or pamoate salts.
The studies included these laboratory determinations: complete blood counts (including hemoglobin and hematocrit determinations), urinalyses (including microscopic examination), serum alkaline phosphatase, serum bilirubin, and bromsulphalein retention. The studies also included records of weight and blood pressure and, in one, electrocardiograms.
In two of these studies, clinical laboratory changes were seen among volunteers in both treated and control groups. The changes included: extrasystoles, white cells in the urine, increase in prothrombin time, rise in hematocrit, rise in leucocytes, rise in eosinophils, and rise or reduction in neutrophils. At no time in any study did changes in the treated group differ significantly from those in the control group.
‘Vontrol’, as the hydrochloride salt, was given orally to 17 children (aged five to 15). Total daily doses ranged from 90 to 240 mg. Complete blood counts and, in some patients, urinalyses were done before treatment and after approximately four days of treatment. There was no significant difference between pre- and post-treatment laboratory determinations in any child. No side effects were seen.
EXCRETION
Following oral administration of ‘Vontrol’ (diphenidol, SK&F) to dogs, as the hydrochloride or pamoate salts, and to humans, as the hydrochloride salt, peak blood concentration of the drug generally occurs in one and a half to three hours. In dogs and rats, virtually all of an oral dose of C14-labeled ‘Vontrol’ is excreted in the urine and feces within three to four days, as determined by radioactivity counts. Approximately the same percentage of an administered dose appeared in the urine of dogs following either oral administration of the hydrochloride salt or rectal administration of the free base.
IN PREGNANCY
Investigators kept follow-up records on 936 patients who had received ‘Vontrol’ (diphenidol, SK&F) at some time during pregnancy, primarily during the first trimester.
Of the 936 women, 864 (92%) had normal births of normal infants.
Seventy-two (8%) of the women experienced some birth abnormality. Of the 72, six patients had premature but otherwise normal infants, 40 patients aborted, 10 had stillbirths, and 16 had infants with miscellaneous defects. These included hernias, congenital heart defects, hydrocephalus, internal strabismus, anencephalus, enlarged thyroid, and hypospadia.
These incidences of abnormal birth are lower than those generally reported in the literature for the average population of pregnant patients. And in no instance was there any evidence that the administration of ‘Vontrol’ played a part in birth abnormality.
DATE OF ISSUANCE JUNE 1985
© SmithKline Beckman Corporation, 1980
Smith Kline &French Laboratories Division of SmithKline Beckman Corporation Philadelphia, Pa. 19101
VN:L15 Printed in U.S.A.
| VONTROL
diphenidol tablet |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - SmithKline Beckman Corporation |