Podocon-25 Prescribing Information
Package insert / product label
Generic name: podophyllum resin in benzoin tincture
Dosage form: tincture
Drug class: Topical keratolytics
Medically reviewed by Drugs.com. Last updated on Nov 14, 2023.
On This Page
Podocon-25 Description
Podocon-25® is composed of Podophyllin (Podophyllum Resin, American) 25% in Benzoin Tincture. Podophyllum Resin is the powdered mixture of resins removed from the May apple or Mandrake (PodophyllumpeltatumLinne’ ), a perennial plant of northern and middle United States(1). The podophyllin resin used in this product is exclusively the American podophyllin (rather than the Indian resin). American podophyllin typically has a reduced level of podophyllotoxin (see below).
Podocon-25 - Clinical Pharmacology
Podophyllin is a cytotoxic agent that has been used topically in the treatment of genital warts. It arrests mitosis in metaphase, an effect it shares with other cytotoxic agents such as the vinca alkaloids(2). The active agent is podophyllotoxin, whose concentration varies with the type of podophyllin used; the American source normally containing one-fourth the amount of podophyllotoxin as the Indian source(3).
NOTE: PODOCON-25® IS TO BE APPLIED ONLY BY A PHYSICIAN. IT IS NOT TO BE DISPENSED TO THE PATIENT.
Indications and Usage for Podocon-25
Podocon-25® (25% podophyllin in benzoin tincture) is indicated for the removal of soft genital (venereal) warts (condylomata acuminata)(4).
Contraindications
Podocon-25® is contraindicated in diabetics, patients using steroids or with poor blood circulation. Podocon-25® should not be used on bleeding warts, moles, birthmarks or unusual warts with hair growing from them. It is recommended that Podocon- 25® not be used during pregnancy (see Pregnancy warning below).
Warnings
Podophyllin is a powerful caustic and severe irritant. Keep away from the eyes; if eye contact occurs, flush with copious amounts of warm water and consult physician or poison control center immediately for advice.
Precautions
Do not use Podocon- 25® if wart or surrounding tissue is inflamed or irritated. Do not use on bleeding warts, moles, birthmarks or unusual warts with hair growing from them.
Adverse Reactions/Side Effects
The use of topical podophyllin has been known to result in paresthesia, polyneuritis, paralytic ileus, pyrexia, leukopenia, thrombocytopenia, coma and death(5).
Pregnancy:
There have been reports of complications associated with the topical use of podophyllin on condylomata of pregnant patients including birth defects, fetal death and stillbirth (6). In the absence of controlled safety studies, podophyllin remains contraindicated for use on pregnant patients.
Podocon-25 Dosage and Administration
PODOCON-25® IS TO BE APPLIED ONLY BY A PHYSICIAN. IT IS NOT TO BE DISPENSED TO THE PATIENT. SHAKE WELL. Thoroughly cleanse affected area. Use supplied applicator to apply Podocon-25® sparingly to lesion. Avoid contact with healthy tissue. Allow to dry thoroughly. Only intact (no bleeding) lesions should be treated. As podophyllin is a powerful caustic and severe irritant, it is recommended the first application of Podocon-25® be left in contact for only a short time (30-40 minutes) to determine patient’s sensitivity. To avoid systemic absorption, time of contact should be minimum time necessary to produce the desired result (1 to 4 hours, depending on condition of lesion and of patient), the physician developing his/her own experience and technique. Large areas or numerous warts should not be treated at once.
After treatment time has elapsed, remove dried Podocon-25® thoroughly with alcohol or soap and water.
How is Podocon-25 supplied
Podocon-25® is available in 15-mL bottles with tapered tip applicator attached inside cap. NDC 0574-0601-15
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature] in tight, light-resistant containers.
Rx Only
1) Blumgarten, A.F.: Text Book of Materia Medica, Pharmacology and Therapeutics; Ed. 7, New York, The Macmillan Company, 1937, pp. 220 and 223.
2) Green, L.K., Klima, M., Burns, T.; Arch Dermatol. Vol 124, Nov 1988, p. 1718.
3) Martindale, 28th Ed. London, 1982, pp. 1366, 1367.
4) Medical Letter; Vol 26, New Rochelle, N.Y., 1984, p10.
5) Fisher: Severe Systemic and Local Reactions to Topical Podophyllum Resins; Cutis, Volume 28, 1981.
6) Zackheim: Hazards of Topical Mitotic-Blocking Agents; Arch. Dermat. Volume 113, 1977.
Manufactured By
Perrigo®
Minneapolis, MN 55427
2124075
(05-12)
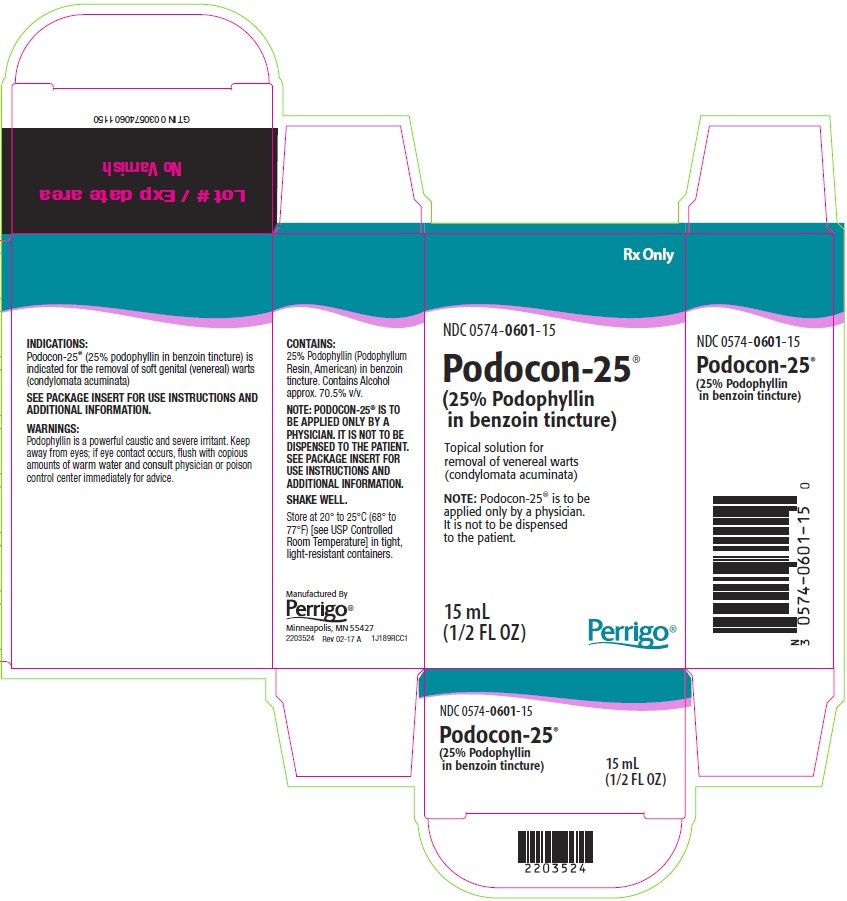
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
Rx Only
NDC 0574-0601-15
Podocon-25®
(25% Podophyllin in benzoin tincture)
Topical solution for removal of venereal warts (condylomata acuminata)
NOTE: Podocon-25® is to be applied only by a physician. It is not to be dispensed to the patient.
15 mL
(1/2 FL OZ)
The following image is a placeholder representing the product identifier that is either affixed or imprinted on the drug package label during the packaging operation.
| PODOCON 25
podophyllum resin tincture |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Padagis US LLC (967694121) |
More about Podocon-25 (podophyllum resin topical)
- Compare alternatives
- Pricing & coupons
- Side effects
- Dosage information
- Drug class: topical keratolytics
- En español