Balsalazide Tablets Prescribing Information
Package insert / product label
Generic name: balsalazide disodium
Dosage form: tablet
Drug class: 5-aminosalicylates
Medically reviewed by Drugs.com. Last updated on Mar 25, 2024.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- References
- How Supplied/Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
BALSALAZIDE disodium tablets, for oral use
Initial U.S. Approval: 2000
Indications and Usage for Balsalazide Tablets
Balsalazide Disodium Tablets is a locally acting aminosalicylate indicated for the treatment of mildly to moderately active ulcerative colitis in male patients 18 years of age and older. (1) (1)
Limitations of Use (1)
- Effectiveness in female patients was not demonstrated in clinical trials. (1)
- Safety and effectiveness of Balsalazide Disodium Tablets beyond 8 weeks have not been established. (1)
Balsalazide Tablets Dosage and Administration
Three 1.1 g Balsalazide Disodium Tablets 2 times a day (6.6 g/day) with or without food for up to 8 weeks. (2) (2)
Dosage Forms and Strengths
Tablets: 1.1 g (3) (3)
Contraindications
Patients with hypersensitivity to salicylates or to any of the components of balsalazide disodium tablets or balsalazide metabolites (4) (4)
Warnings and Precautions
- Exacerbation of the symptoms of ulcerative colitis was reported. Observe patients closely for worsening of these symptoms while on treatment. (5.1)
- Renal impairment may occur. Assess renal function at the beginning of treatment and periodically during treatment. (5.2)
- Use with caution with pre-existing liver disease. (5.3)
Adverse Reactions/Side Effects
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 2/2016
Related/similar drugs
Entyvio, prednisone, methylprednisolone, dexamethasone, budesonide, hydrocortisone topical, Humira
Full Prescribing Information
1. Indications and Usage for Balsalazide Tablets
Balsalazide Disodium Tablets is indicated for the treatment of mildly to moderately active ulcerative colitis in male patients 18 years of age and older.
Limitations of Use:
- Effectiveness of Balsalazide Disodium Tablets in the treatment of female patients was not demonstrated in clinical trials [see Clinical Trials (14.1)].
- Safety and effectiveness of Balsalazide Disodium Tablets therapy beyond 8 weeks have not been established.
2. Balsalazide Tablets Dosage and Administration
The dose is three 1.1 g Balsalazide Disodium Tablets to be taken 2 times a day with or without food (6.6 g per day) for up to 8 weeks.
3. Dosage Forms and Strengths
Balsalazide Disodium Tablets is available as yellow colored, oval shaped, biconvex, film-coated tablets debossed with “P” on one side and “840” on other side.
4. Contraindications
Balsalazide Disodium Tablets is contraindicated in patients with hypersensitivity to salicylates, aminosalicylates or their metabolites, or to any of the components of balsalazide disodium Tablets [see Description (11)].
5. Warnings and Precautions
5.1 Exacerbations of Ulcerative Colitis
Balsalazide is converted to mesalamine, which has been associated with an acute intolerance syndrome that may be difficult to distinguish from an exacerbation of ulcerative colitis. In controlled clinical trials with balsalazide disodium in adults with ulcerative colitis, 7% of male patients reported exacerbation of the symptoms of ulcerative colitis. Symptoms include cramping, acute abdominal pain and bloody diarrhea, sometimes fever, headache, and rash. Observe patients closely for worsening of these symptoms while on treatment. If acute intolerance syndrome is suspected, promptly discontinue treatment with balsalazide disodium.
5.2 Renal Impairment
Renal impairment, including minimal change nephropathy, acute and chronic interstitial nephritis and renal failure, has been reported in patients given products that release mesalamine in the gastrointestinal tract. Evaluate renal function prior to initiation of balsalazide disodium tablets therapy and periodically while on therapy. Exercise caution when using balsalazide disodium tablets in patients with known renal dysfunction or a history of renal disease.
5.3 Use in Hepatic Impairment
There have been reports of hepatic failure in patients with pre-existing liver disease who have been administered mesalamine. Because balsalazide is converted to mesalamine, use caution and consider liver function testing when administering balsalazide disodium to patients with liver disease.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure of balsalazide disodium in 565 ulcerative colitis patients with mildly to moderately active disease. Balsalazide disodium was evaluated in one placebo-controlled trial (168 treated with balsalazide disodium), one active-controlled trial (210 treated with balsalazide disodium); and a subset of these patients also participated in an uncontrolled, open-label, extension study (additional 187 treated with balsalazide disodium). The population studied had a mean age of 43.1 (range: 18 to 80) years; approximately 94% of patients were < 65 years old, 49% were male, and 84% were white.
In the placebo-controlled trial, the most common adverse reactions with balsalazide disodium in male patients were headache, nasopharyngitis, anemia, diarrhea, fatigue, pharyngolaryngeal pain, and urinary tract infection. 10% of patients in the balsalazide disodium group and 13% of patients in the placebo group discontinued treatment due to an adverse reaction. The majority of adverse reactions were mild to moderate in severity. The most common serious adverse reactions in both the placebo and balsalazide disodium groups were gastrointestinal disorders, which were mainly associated with symptoms of ulcerative colitis.
Adverse reactions occurring in at least 2% of male patients and at a rate numerically higher than placebo in the placebo-controlled trial are listed in Table 1.
Table 1: Adverse Reactions Experienced by at Least 2% of Balsalazide Disodium –Treated Male
Patients and at a Rate Numerically Greater than Placebo in a Placebo-Controlled Trial
| Balsalazide Disodium 6.6 g/day
| Placebo
|
|
| Adverse Reaction
| N=82
| N=37
|
| Anemia | 3.7% | 0% |
| Diarrhea | 3.7% | 0% |
| Pharyngolaryngeal Pain | 3.7% | 0% |
| Urinary Tract Infection | 3.7% | 0% |
| Arthralgia | 2.4% | 0% |
| Insomnia | 2.4% | 0% |
| Musculoskeletal Pain | 2.4% | 0% |
Data collected from all three trials (placebo-controlled, active-controlled, and open-label) showed that female patients reported adverse reactions more frequently than did male patients (76% and 66%, respectively).
The following adverse reactions, presented by body system, were reported by less than 1% of balsalazide disodium-treated ulcerative colitis patients in controlled trials.
Cardiovascular and Vascular: increased blood pressure, increased heart rate
Dermatological: erythema nodosum, rash
Respiratory, Thoracic and Mediastinal Disorders: dyspnea
Gastrointestinal Disorders: abdominal pain, constipation, defecation urgency, diarrhea, dry mouth, hard feces, flatulence, gastroesophageal reflux disease, vomiting
Hepatobiliary Disorders: increased aspartate aminotransferase
Infections and Infestations: gastroenteritis, upper respiratory infection
Musculoskeletal and Connective Tissue Disorders: arthralgia, back pain, myalgia
Nervous System Disorders: dizziness, lethargy
General Disorders and Administrative Site Disorders: face edema, fatigue, malaise, pain, pyrexia, swelling
6.2 Postmarketing Experience
Because these reactions are reported voluntarily from a population of unknown size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These adverse reactions have been chosen for inclusion due to a combination of seriousness, frequency of reporting, or potential causal connection to products which contain or are metabolized to mesalamine, including balsalazide.
Cardiovascular and Vascular: myocarditis, pericarditis, vasculitis
Respiratory: alveolitis, pleural effusion, pneumonia (with and without eosinophilia)
Gastrointestinal: pancreatitis
Renal: interstitial nephritis, renal failure.
Hepatobiliary Disorders: elevated liver enzymes (AST, ALT, GGT, LDH, alkaline phosphatase), elevated bilirubin, jaundice, cholestatic jaundice, cirrhosis, hepatocellular damage including liver necrosis and liver failure, Kawasaki-like syndrome including hepatic dysfunction. Some of these cases were fatal.
Dermatological: alopecia, pruritus
7. Drug Interactions
Based on in vitro studies, balsalazide and its metabolites [5-aminosalicylic acid (5-ASA), N-acetyl-5-aminosalicylic acid (N-Ac-5-ASA), 4-aminobenzoyl-ß-alanine (4-ABA), and N-acetyl-4-aminobenzoyl-ß-alanine (N-Ac-4-ABA)] are not expected to inhibit the metabolism of other drugs that are substrates of CYP1A2, CYP2C9, CYP2C19, CYP2D6, or CYP3A4/5.
8. Use In Specific Populations
8.1 Pregnancy
Pregnancy Category B. Reproduction studies were performed in rats and rabbits at oral doses up to 2 g/kg/day, 2.5 and 4.9 times the recommended human dose based on body surface area for the rat and rabbit, respectively, and revealed no evidence of impaired fertility or harm to the fetus due to balsalazide disodium. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Mesalamine, a metabolite of balsalazide disodium, is known to cross the placental barrier.
8.3 Nursing Mothers
It is not known whether balsalazide disodium or its metabolites are excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when balsalazide disodium is administered to a nursing woman.
8.4 Pediatric Use
Safety and effectiveness of balsalazide disodium in pediatric patients have not been established.
8.5 Geriatric Use
Reports from uncontrolled clinical studies and postmarketing reporting systems suggested a higher incidence of blood dyscrasias, i.e., neutropenia and pancytopenia, in patients who were 65 years or older who were taking mesalamine-containing products. Balsalazide disodium is converted into mesalamine in the colon. Caution should be taken to closely monitor blood cell counts during therapy.
Clinical trials of balsalazide disodium did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently than younger subjects. Other reported clinical experience has not identified differences in responses between elderly and younger patients. In general, the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy in elderly patients should be considered when prescribing balsalazide disodium.
10. Overdosage
No case of overdose has been reported with balsalazide disodium tablets. Balsalazide disodium is an aminosalicylate, and symptoms of salicylate toxicity include: hematemesis, tachypnea, hyperpnea, tinnitus, deafness, lethargy, seizures, confusion, or dyspnea. Severe intoxication may lead to electrolyte and blood pH imbalance and potentially to other organ (e.g., renal and liver) involvement. There is no specific antidote for balsalazide overdose. Proper medical care should be sought immediately with appropriate supportive care, including the possible use of emesis, cathartics, and activated charcoal to prevent further absorption.
11. Balsalazide Tablets Description
Each Balsalazide Disodium Tablet contains 1.1 g of balsalazide disodium, an orally available prodrug that is enzymatically cleaved to produce mesalamine (5-aminosalicylic acid, 5-ASA), an anti-inflammatory drug. Balsalazide disodium has the chemical name (E)-5-[[-4-[[(2-carboxyethyl) amino]carbonyl] phenyl]azo]-2-hydroxybenzoic acid, disodium salt, dihydrate. Its structural formula is:
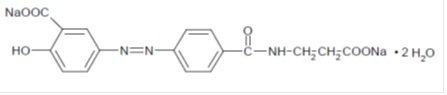
Molecular Weight: 437.32
Molecular Formula: C17H13N3O6Na2•2H2O
Balsalazide disodium is a stable, odorless, yellow to orange crystalline powder. It is insoluble in acid, but soluble at a pH of at least 4.5. It is freely soluble in water and isotonic saline, sparingly soluble in methanol and ethanol, and practically insoluble in all other organic solvents.
Inactive Ingredients: Each tablet contains d&c yellow#10 aluminum lake, fd&c yellow#6/sunset yellow fcf aluminum lake, hypromellose, macrogol, magnesium stearate, polydextrose, titanium dioxide and triacetin. The sodium content of each tablet is approximately 126 mg
12. Balsalazide Tablets - Clinical Pharmacology
12.1 Mechanism of Action
Balsalazide is a prodrug of mesalamine (5-aminosalicylic acid, 5-ASA). The mechanism of action of 5-ASA is unknown, but appears to be local to the colonic mucosa rather than systemic. Mucosal production of arachidonic acid metabolites, both through the cyclooxygenase pathways, i.e., prostanoids, and through the lipoxygenase pathways, i.e., leukotrienes and hydroxyeicosatetraenoic acids, is increased in patients with ulcerative colitis, and it is possible that 5-ASA diminishes inflammation by blocking production of arachidonic acid metabolites in the colon.
12.3 Pharmacokinetics
Following oral administration, balsalazide is cleaved by azoreductases produced by anaerobic bacteria found in the gut, to release equimolar quantities of 5-ASA, the active moiety, and 4-aminobenzoyl-ß-alanine (4-ABA), a carrier moiety. Both of these moieties are N-acetylated to form N-Ac-5-ASA and N-Ac-4-ABA, respectively.
Absorption
After single-dose administration of 3.3 g balsalazide disodium tablets in 18 healthy subjects, the median time of peak plasma concentration (Tmax) was 0.5 hr for balsalazide, while the median Tmax was 12 hr for both 5-ASA and N-Ac-5-ASA (Table 2). Pharmacokinetic parameters exhibited high variability, with %CV ranging from 31% to 67% for AUC and from 27% to 68% for Cmax.
Pharmacokinetics were also estimated in healthy volunteers after repeated doses of 3.3 g balsalazide disodium tablets every 12 hours for 7 days. After multiple doses, steady-state was achieved after about 3 days for balsalazide and all metabolites. The AUC and Cmax were the highest for N-Ac-5-ASA, followed by 5-ASA and balsalazide. There was minimal accumulation of balsalazide, as suggested by a 1.2-fold increase in AUC; however, a relatively larger increase in the systemic exposure to metabolites was observed at steady-state. The accumulation ratios based on AUC for the metabolites were 6.1 for 5-ASA, 3.6 for N-Ac-5-ASA, 4.8 for 4-ABA, and 3.6 for N-Ac-4-ABA.
Table 2: Pharmacokinetic Parameters for Balsalazide and Metabolites (5-ASA and N-Ac-5-ASA) Following Single-and Repeated-Doses (Q12) of 3.3 g Balsalazide Disodium as Balsalazide Disodium Tablets (N=18)
| Single Dose
| Repeated Dose
|
||||
| Parameter
| Mean
| SD
| Mean
| SD
|
|
| Cmax (mcg/mL)
| |
||||
| Balsalazide | 0.3 | 0.2 | 0.3 | 0.2 |
|
| 5-ASA | 0.5 | 0.3 | 1.5 | 0.6 |
|
| N-Ac-5-ASA | 1.2 | 0.4 | 2.2 | 0.6 |
|
| Tmaxa (hours)
| |
||||
| Balsalazide | 0.5 | (0.5-2) | 0.5 | (0.5-2) |
|
| 5-ASA | 12 | (8-16) | 12 | (1.5-16) |
|
| N-Ac-5-ASA | 12 | (8-16) | 10 | (1-16) |
|
| AUCtau (mcg•h/mL)
| |
||||
| Balsalazide | 1.3 | 0.7 | 1.6 | 0.9 |
|
| 5-ASA | 2.2 | 1.6 | 13.4 | 6.3 |
|
| N-Ac-5-ASA | 5.9 | 2.9 | 21 | 6.4 |
|
| AUC0-∞ (mcg•h/mL)
| |
||||
| Balsalazide | 1.4 | 0.8 | NA | NA |
|
| 5-ASA | 8.5 | 3.9 | NA | NA |
|
| N-Ac-5-ASA | 33.5 | 14.1 | NA | NA |
|
| T½b (hour)
| |
||||
| Balsalazide | 1.9 | 0.7 | 8.4 | 12.4 |
|
| 5-ASA | 9.5b
| 10.1 | 9.0 | 8.6 |
|
| N-Ac-5-ASA | 10.4b
| 17.6 | 7.2 | 6.8 |
|
a Expressed as median and range.
b N=17
Food effect
After administration of single dose of 3.3 g (3 × 1.1 g tablets) of balsalazide disodium tablets with a high-fat meal in healthy volunteers, the AUC of balsalazide was unaffected compared to fasted administration, but the presence of food reduced both peak concentrations and AUC of the metabolites 5-ASA and N-Ac-5-ASA. A high fat meal increased the median Tmax for balsalazide from 0.5 to 2 hours; for 5-ASA from 12 to 24 hours; and for N-Ac-5-ASA from 12 to 24 hours. Under fed conditions, the mean Cmax was reduced by 44% for balsalazide, 65% for 5-ASA, and 48% for N-Ac-5-ASA. No significant changes were observed for AUC0 to ∞ for balsalazide; however, AUC0 to ∞ was reduced for 5-ASA by 46% and for N-Ac-5-ASA by 17%.
Distribution
The binding of balsalazide to human plasma proteins was ≥ 99%; 5-ASA and N-Ac-5-ASA were 43% and 78% bound, respectively, to plasma proteins.
Metabolism and Excretion
Following oral administration, balsalazide is cleaved by bacterial azoreduction to release equimolar quantities of 5-ASA, the active moiety, and 4-ABA, a carrier moiety. Mesalamine (5-ASA) and 4-ABA are further acetylated to N-Ac-5-ASA and N-Ac-4-ABA, respectively in the intestinal mucosa and liver. The terminal half-life was 1.9 h for balsalazide, 9.5 h for 5-ASA, and 10.5 h for N-Ac-5-ASA.
At steady-state following administration of repeated doses of 3.3 g balsalazide disodium tablets every 12 hours in healthy volunteers, the combined % of dose excreted in urine for balsalazide and its metabolites over 12 hours was 23%. The mean % of dose excreted in urine over 12 hours was 0.16% for balsalazide, 4.6% for 5-ASA, 15.6% for N-Ac-5-ASA, 0.40% for 4-ABA, and 1.8% for N-Ac-4-ABA.
13. Nonclinical Toxicology
13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility
In a 24-month rat (Sprague Dawley) carcinogenicity study, oral (dietary) balsalazide disodium at doses up to 2 g/kg/day was not tumorigenic. For a 50 kg person of average height this dose represents 2.5 times the recommended human dose on a body surface area basis. Balsalazide disodium was not genotoxic in the following in vitro or in vivo tests: Ames test, human lymphocyte chromosomal aberration test, and mouse lymphoma cell (L5178Y/TK+/-) forward mutation test, or mouse micronucleus test. However, it was genotoxic in the in vitro Chinese hamster lung cell (CH V79/HGPRT) forward mutation test.
The compound 4-aminobenzoyl-ß-alanine, a metabolite of balsalazide disodium, was not genotoxic in the Ames test and the mouse lymphoma cell (L5178Y/TK+/-) forward mutation test but was positive in the human lymphocyte chromosomal aberration test. N-acetyl-4-aminobenzoyl-ß-alanine, a conjugated metabolite of balsalazide disodium, was not genotoxic in Ames test, the mouse lymphoma cell (L5178Y/TK+/-) forward mutation test, or the human lymphocyte chromosomal aberration test. Balsalazide disodium at oral doses up to 2 g/kg/day, 2.5 times the recommended human dose based on body surface area, was found to have no effect on fertility and reproductive performance in rats.
14. Clinical Studies
14.1 Ulcerative Colitis
A double-blind, placebo-controlled, multi-center trial was conducted in 250 adult patients with mildly to moderately active ulcerative colitis. The trial population was primarily white (84%), had a mean age of 44 years (7% age 65 years or older), and 49% were men. Disease activity was assessed using a modified Mayo Disease Activity Index1 (MMDAI), which was a sum of four subscores (bowel frequency, rectal bleeding, endoscopic appearance, and physician’s global assessment), each ranging from 0 to 3, with higher scores indicating worse disease. The median baseline MMDAI score was 8 and the median baseline rectal bleeding subscore was 2. Patients were randomized 2:1 to receive 8 weeks of treatment with either balsalazide disodium tablets 3.3 g twice daily or placebo.
The primary efficacy endpoint was the proportion of patients that achieved clinical improvement and improvement in the rectal bleeding subscale of the MMDAI at the end of 8 weeks of treatment. Clinical Improvement was defined as having both a ≥ 3 point improvement from baseline in the MMDAI score and a ≥ 1 point improvement from baseline in the rectal bleeding subscore. Two key secondary efficacy endpoints were the proportion of patients with Clinical Remission and Mucosal Healing at the end of 8 weeks of treatment. Clinical Remission was defined as a score of 0 for rectal bleeding and a combined score of ≤ 2 for bowel frequency and physician’s assessment using the MMDAI subscale; the endoscopic sub-score was not considered in this definition. Mucosal Healing was defined as an endoscopy/sigmoidoscopy score of 0 or 1, where a score of 1 could include signs of erythema or decreased vascular pattern; by definition, the presence of friability indicated a score of 2 or 3.
After 8 weeks of treatment, the proportion of patients who met the definition of Clinical Improvement was greater for the balsalazide disodium tablets-treated group compared to the placebo group (Table 3).
Table 3: Proportion of Patients with Clinical Improvement* at Week 8
for the Total Population and by Gender Subgroups
| Balsalazide Disodium Tablets
| Placebo
| p-value |
|
| Total Population
| 55%
| 40%
| 0.0237 |
| Males | 57%
| 20% | |
| Females | 54%
| 58% |
* Clinical Improvement: ≥ 3 improvement in MMDAI score and ≥ 1 point improvement in rectal bleeding.
These differences were statistically significant in the overall population; however, these effects were entirely driven by the results in the male subpopulation. With adjustment for multiplicity, statistically significant differences were also seen in the male patients for Clinical Remission (35% with balsalazide disodium tablets vs. 13% for placebo) and for Mucosal Healing (52% with balsalazide disodium tablets vs. 20% for placebo). Effectiveness of balsalazide disodium tablets was not demonstrated in the female subpopulation in the clinical trial.
15. References
1. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mild to moderately active ulcerative colitis: a randomized study. N Engl J Med. 1987;317:1625-9.
16. How is Balsalazide Tablets supplied
How Supplied
Balsalazide Disodium Tablets are available as yellow colored, oval shaped, biconvex, film-coated tablets debossed with “P” on one side and “840” on other side.
NDC 49884-367-13 Bottles of 180 tablets
NDC 49884-367-05 Bottles of 500 tablets
Storage
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F). See USP Controlled Room Temperature.
17. Patient Counseling Information
- Instruct patients not to take balsalazide disodium tablets if they have a hypersensitivity to salicylates (e.g., aspirin).
- Instruct patients to take balsalazide disodium tablets with food.
- Advise patients who need to control sodium intake that the recommended dosing of balsalazide disodium tablets (6.6 g/day) provides about 756 mg of sodium per day.
- Instruct patients to contact their health care provider if they experience a worsening of their ulcerative colitis symptoms, because it could be due to a reaction to balsalazide disodium tablets.
- Instruct patients to make sure they let their health care provider know:
- If they have or are later diagnosed with renal dysfunction. Damage to the kidney has been observed in some people given medications similar to balsalazide disodium tablets.
- If they have or are later diagnosed with liver disease. Worsening liver disease has been observed in some people given medications similar to balsalazide disodium tablets.
- If they have or are later diagnosed with pyloric stenosis, because balsalazide disodium tablets may be slow to pass through their digestive tract.
Distributed by:
Par Pharmaceutical Companies, Inc.
Chestnut Ridge, NY 10977 U.S.A.
Manufactured by:
Par Formulations Private Limited,
1/58, Pudupakkam,
Kelambakkam - 603 103.
Made in India
Mfg. Lic. No.: TN00002121
OS367-01-74-01
SE7528/00
I04/15
| BALSALAZIDE DISODIUM
balsalazide disodium tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Par Pharmaceutical Inc. (092733690) |
| Registrant - Par Pharmaceutical Inc. (092733690) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Par Formulations Private Limited | 676159161 | MANUFACTURE(49884-367) | |
More about balsalazide
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (34)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: 5-aminosalicylates
- Breastfeeding
- En español