Ammonia Inhalants Prescribing Information
Package insert / product label
Dosage form: inhalant
Active ingredient(s)
Ammonia (15%)
Indications and Usage for Ammonia Inhalants
To prevent or treat fainting
Warnings
Keep away from the Eyes.
Stop use and ask a doctor if
condition persists
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Ammonia Inhalants Dosage and Administration
Directions: hold inhalant away from face and crush between thumb and forefinger. Carefully approach crushed inhalant to nostrils of affected person.
Other information
Store at room temperature away from light.
Storage and Handling
Store at 20ºC to 25ºC (68ºF to 77ºF)
Inactive ingredients
Alcohol USP, FDC red dye 40, lavender oil fcc, lemon oil fcc, nutmeg oil fcc, purified water usp
Questions
Questions? Call 1-866-390-4411 Mon - Fri 9:00 AM - 5:00 PM
Principal Display Panel

Ampule Label
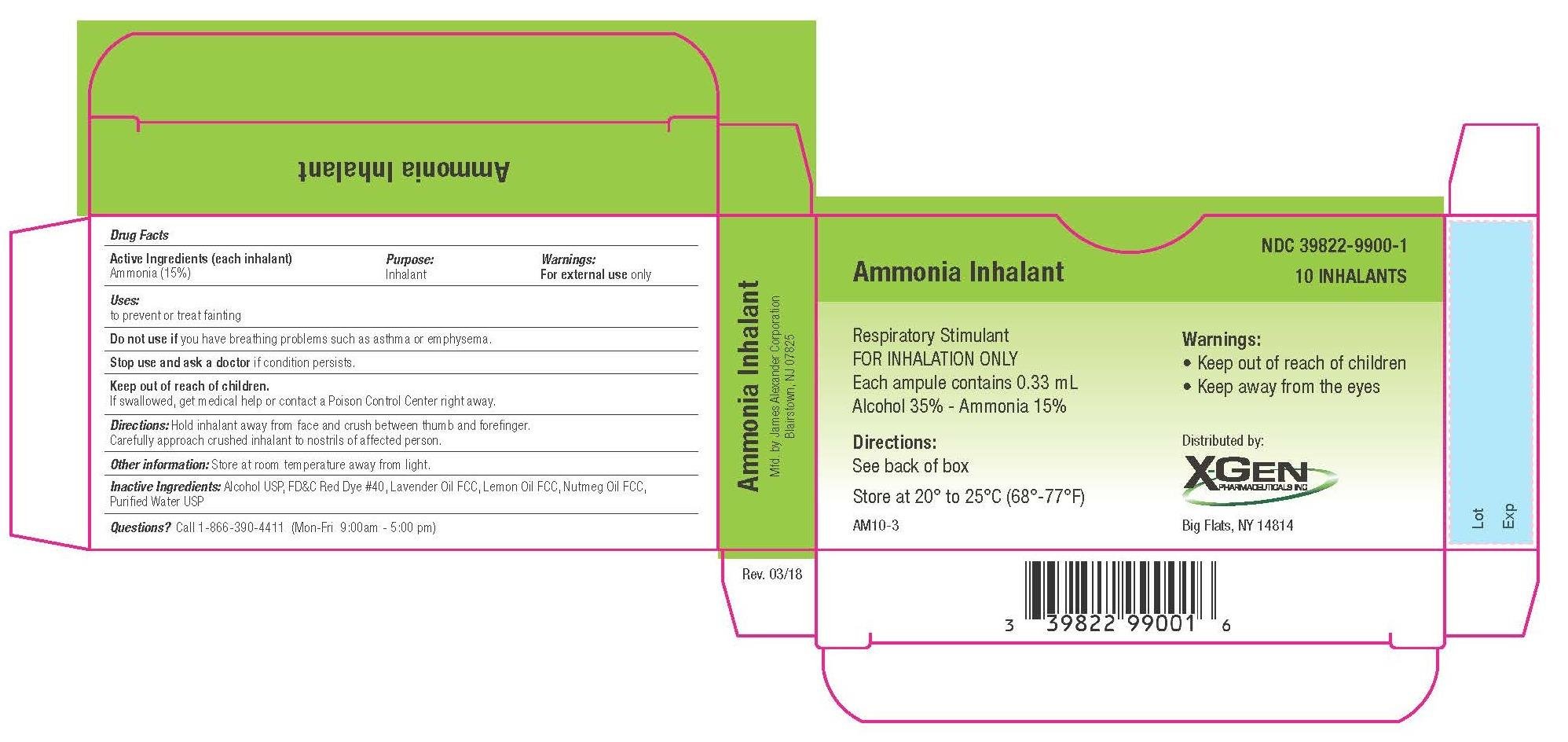
10 pack carton

12 pack carton
AMMONIA INHALANTS
ammonia inhalants inhalant |
|
|
|
|
|
|
|
|
|
|
|
|
Medical Disclaimer



