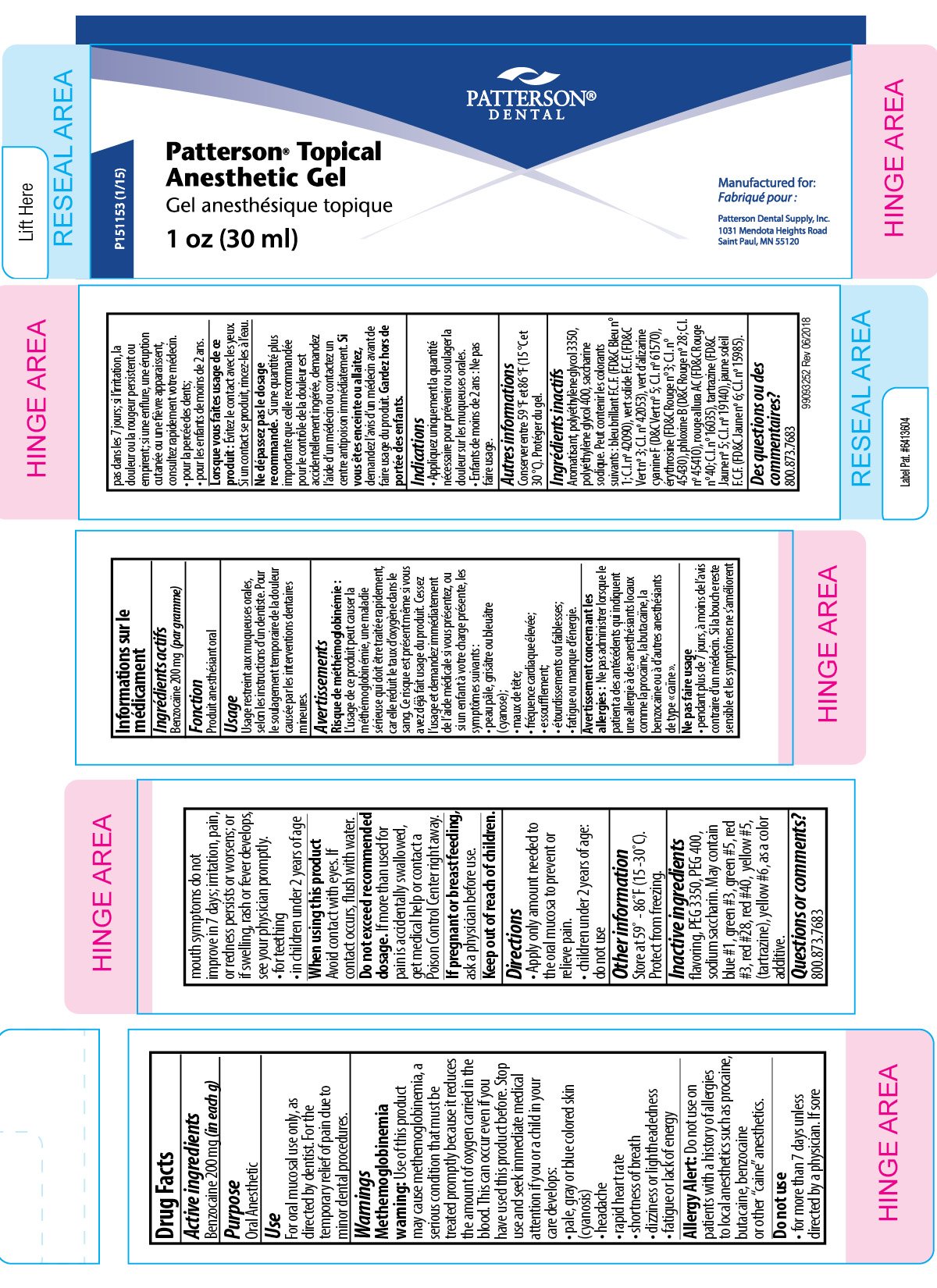
Patterson Dental Topical Anesthetic
Dosage form: gel
Ingredients: BENZOCAINE 200mg in 1g
Labeler: Patterson Dental
NDC code: 50227-1009
Medically reviewed by Drugs.com. Last updated on Dec 4, 2023.
Benzocaine 200mg (in each g)
Oral Anesthetic
For oral mucosal use only, as directed by dentist. For the temporary relief of pain due to minor dental procedures.
Methemoglobinemia warning: Use of this product may cause methemoglobinemia, a serious condition that must be treated promptly because it reduces the amount of oxygen carried in the blood. This can occur even if you have used this product before. Stop use and seek immediate medical attention if you or a child in your care develops:
- pale, gray or blue colored skin (cyanosis)
- headache
- rapid heart rate
- shortness of breath
- dizziness or lightheadedness
- fatigue or lack of energy
Allergy Alert: Do not use on patients with a history of allergies to local anesthetics such as procaine, butacaine, benzocaine or other "caine" anesthetics.
Do not use
- for more than 7 days unless directed by a physician. If sore mouth symptoms do not improve in 7 days; irritation, pain, or redness persists or worsens; or if swelling, rash or fever develops, see your physician promptly.
- for teething
- in children under 2 years of age
When using this product Avoid contact with eyes. If contact occurs, flush with water.
Do not exceed recommended dosage. If more than used for pain is accidentally swallowed, get medical help or contact a Poison Control Center right away.
If pregnant or breast feeding, ask a physician before use.
- Apply only amount needed to the oral mucosa to prevent or relieve pain.
- children under 2 years of age: do not use
Keep out of reach of children.
Store at 59°-86°F (15°-30°C). Protect from freezing.
flavoring, PEG 3350, PEG 400, sodium saccharin. May contain blue #1, green #3, green #5, red #3, red #28, red #40, yellow #5, (tartrazine), yellow #6, as a color additive.
800.873.7683
| PATTERSON DENTAL TOPICAL ANESTHETIC
benzocaine gel |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Patterson Dental (171843584) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.