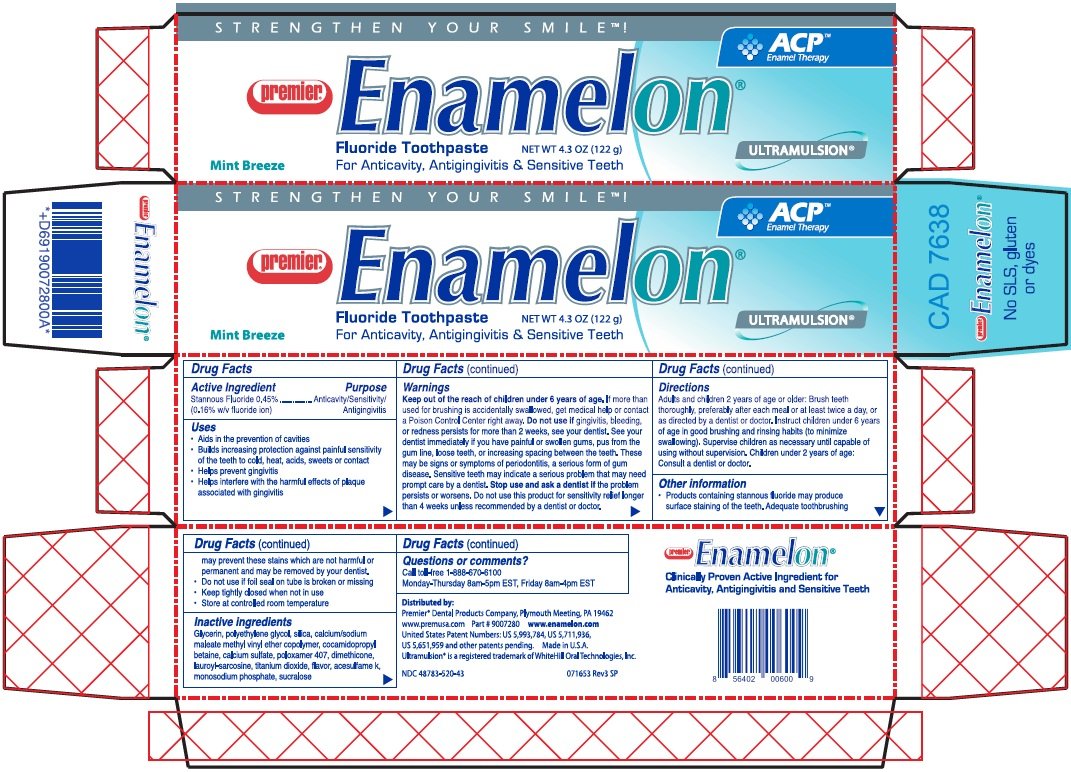
Enamelon
Dosage form: paste, dentifrice
Ingredients: STANNOUS FLUORIDE 0.00454g in 1g
Labeler: Premier Dental Products Company
NDC code: 48783-520
Medically reviewed by Drugs.com. Last updated on Dec 20, 2023.
Stannous fluoride 0.45% (0.16% w/v fluoride ion)
Anticavity/Sensitivity Relief/Antigingivitis
- Aids in the prevention of cavities
- Builds increasing protection against painful sensitivity of the teeth to cold, heat, acids, sweets or contact
- Helps prevent gingivitis
- Helps interfere with the harmful effects of plaque associated with gingivitis
Keep out of the reach of children under 6 years of age.
If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away. Do not use if gingivitis, bleeding, or redness persists for more than 2 weeks, see your dentist. See your dentist immediately if you have painful or swollen gums, pus from the gum line, loose teeth, or increasing spacing between the teeth. These may be signs or symptoms of periodontitis, a serious form of gum disease. Sensitive teeth may indicate a serious problem that may need prompt care by a dentist. Stop use and ask a dentist if the problem persists or worsens. Do not use this product for sensitivity relief longer than 4 weeks unless recommended by a dentist or doctor.
Adults and children 2 years of age or older: Brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or doctor. Instruct children under 6 years of age in good brushing and rinsing habits (to minimize swallowing). Supervise children as necessary until capable of using without supervision. Children under 2 years of age: Consult a dentist or doctor.
- Products containing stannous fluoride may produce surface staining of the teeth. Adequate toothbrushing may prevent these stains which are not harmful or permanent and may be removed by your dentist.
- Do not use if foil seal on tube is broken or missing
- Keep tightly closed when not in use
- Store at controlled room temperature
Glycerin, polyethylene glycol, silica, calcium/sodium maleate methyl vinyl ether copolymer, cocamidopropyl betaine, calcium sulfate, poloxamer 407, dimethicone, lauroyl-sarcosine, titanium dioxide, flavor, acesulfame k, monosodium phosphate, sucralose
Call toll-free 1-888-670-6100
Monday-Thursday 8am-5pm EST, Friday 8am-4pm EST
Premier® Dental Products Company, Plymouth Meeting, PA 19462
www.premusa.com Part # 9007280www.enamelon.com
United States Patent Numbers : US 5,993,784, US 5,711,936, US 5,651,959 and other patents pending. Made in U.S.A.
Ultramulsion® is a registered trademark of WhiteHill Oral Technologies, Inc.
STRENGTHEN YOUR SMILE!
ACPTM Enamel Therapy
Premier®
Enamelon®
Mint Breeze
Fluoride Toothpaste NET WT 4.3 OZ (122 g)
For Anticavity, Antigingivitis & Sensitive Teeth
ULTRAMULSION®
NDC 48783-520-43
| ENAMELON
stannous fluoride paste, dentifrice |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Premier Dental Products Company (014789663) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.