The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
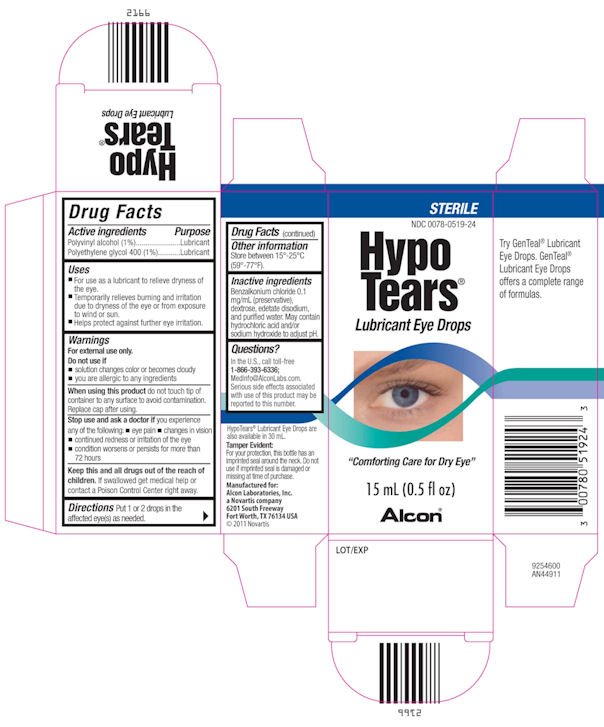
HypoTears
Dosage form: solution/ drops
Ingredients: Polyvinyl Alcohol 10mg in 1mL, Polyethylene Glycol 400 10mg in 1mL
Labeler: Novartis Pharmaceutical Corporation
NDC code: 0078-0519
Polyvinyl alcohol (1%)
Polyethylene glycol 400 (1%)
Lubricant
- For use as a lubricant to relieve dryness of the eye.
- Temporarily relieves burning and irritation due to dryness of the eye or from exposure to wind or sun.
- Helps protect against further eye irritation.
For external use only.
- if solution changes colors or becomes cloudy
- if you are allergic to any ingredients
When using this product do not touch tip of container to any surface to avoid contamination.
Replace cap after using.
Stop use and ask a doctor if you experience any of the following:
- eye pain
- changes in vision
- continued redness or irritation of the eye
- condiiton worsens or persists for more than 72 hours
Keep this and all drugs out of the reach of children. If swallowed get medical help or contact a Poison Control Center right away.
Directions Put 1 or 2 drops in the affected eye(s) as needed.
Store between 15°-25°C (59°-77°F).
Benzalkonium chloride 0.1 mg/mL (preservative), dextrose, edetate disodium, and purified water. May contain hydrochloric acid and/or sodium hydroxide to adjut pH.
In the U.S. call toll-free
1-866-393-6336;
MedInfo@AlconLabs.com.
Serious side effects associated with use of this product may be reported to this number.
| HYPOTEARS
polyvinyl alcohol and polyehtylene glycol 400 solution/ drops |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - Novartis Pharmaceutical Corporation (002147023) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Bausch & Lomb Incorporated | 807927397 | MANUFACTURE(0078-0519) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.