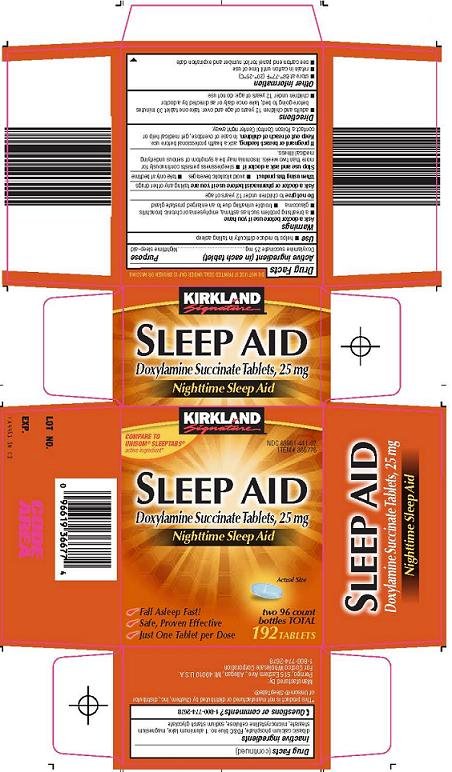
Kirkland Signature Sleep Aid Prescribing Information
Package insert / product label
Generic name: doxylamine succinate
Dosage form: tablet
Drug class: Miscellaneous anxiolytics, sedatives and hypnotics
Medically reviewed by Drugs.com. Last updated on Mar 25, 2024.
Active ingredient (in each tablet)
Doxylamine succinate 25 mg
Purpose
Nighttime sleep-aid
Indications and Usage for Kirkland Signature Sleep Aid
- helps to reduce difficulty in falling asleep
Warnings
Ask a doctor before use if you have
- a breathing problem such as asthma, emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
Do not give
to children under 12 years of age
Ask a doctor or pharmacist before use if you are
taking any other drugs
When using this product
- avoid alcoholic beverages
- take only at bedtime
Stop use and ask a doctor if
- sleeplessness persists continuously for more than two weeks. Insomnia may be a symptom of serious underlying medical illness.
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Kirkland Signature Sleep Aid Dosage and Administration
- adults and children 12 years of age and over: take one tablet 30 minutes before going to bed; take once daily or as directed by a doctor
- children under 12 years of age: do not use
Storage and Handling
- store at 68°-77°F (20°-25°C)
- retain in carton until time of use
- see carton end panel for lot number and expiration date
Inactive ingredients
dibasic calcium phosphate, FD&C blue no. 1 aluminum lake, magnesium stearate, microcrystalline cellulose, sodium starch glycolate
Questions or comments?
1-800-774-2678
Principal Display Panel
Compare to Unisom® SleepTabs® active ingredient
Sleep Aid
Doxylamine Succinate Tablets, 25 mg
Nighttime Sleep Aid
Actual Size
Fall Asleep Fast!
Safe, Proven Effective
Just One Tablet Per Dose
KIRKLAND SIGNATURE SLEEP AID
doxylamine succinate tablet |
|
|
|
|
|
|
|
|
|
|
|
Frequently asked questions
View more FAQ
Medical Disclaimer