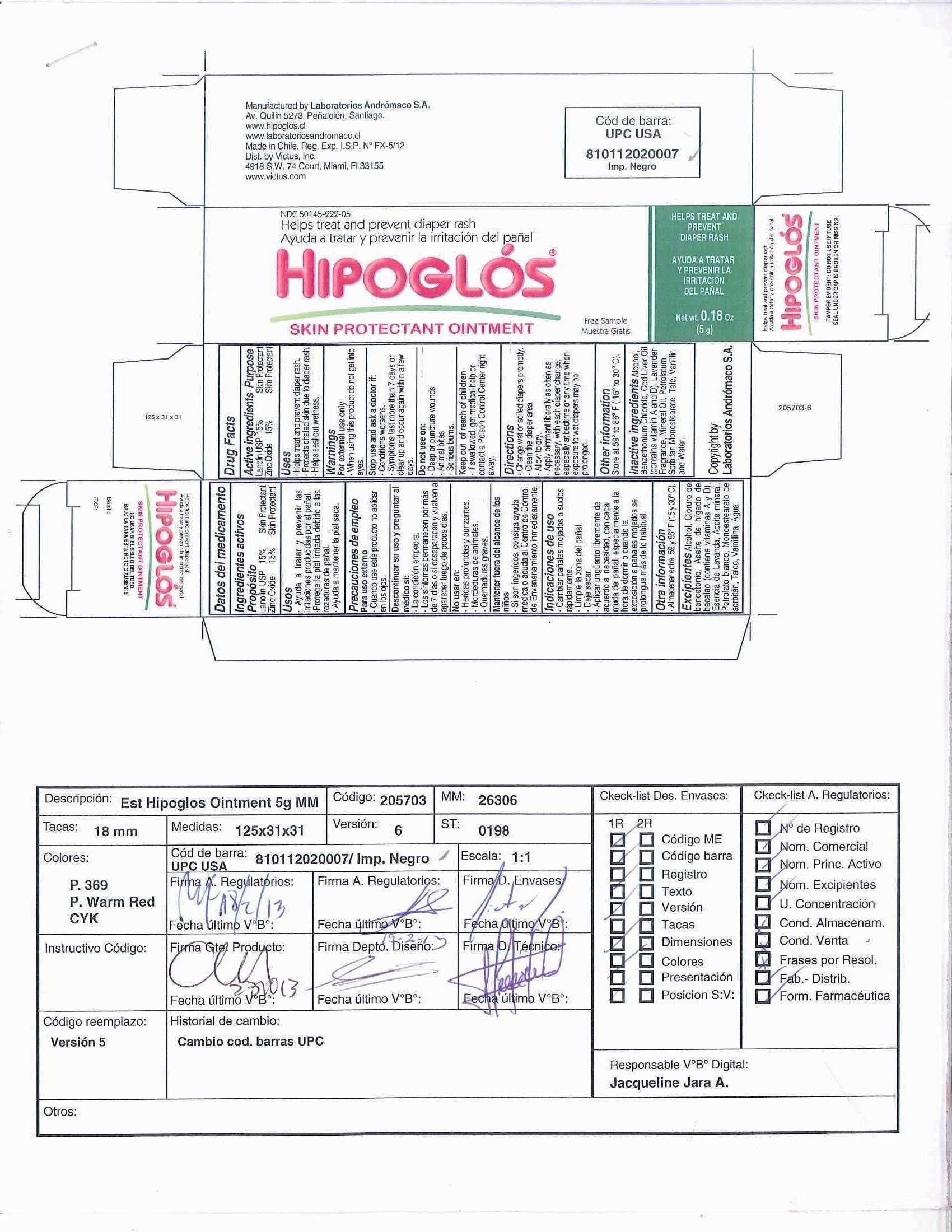
Hipoglos
Dosage form: ointment
Ingredients: ZINC OXIDE 15g in 100g, LANOLIN 15g in 100g
Labeler: Laboratorios Andromaco S.A.
NDC code: 50145-222
Medically reviewed by Drugs.com. Last updated on Oct 23, 2023.
Lanolin USP 15%
Zinc Oxide 15%
Skin Protectant
- Helps treat and prevent diaper rash.
- Protects chafed skin due to diaper rash.
- Helps seal out wetness.
For external use only
When using this product - do not get into eyes
Stop use and ask a doctor if:
- Conditions worsen.
- Symptoms last more than 7 days or clear up and occur again within a few days.
Do not use on:
- Deep or puncture wounds
- Animal Bites
- Serious Burns
- If swallowed, get medical help or contact a Poison Control Center right away.
- Change wet or soiled diapers promptly.
- Clean the diaper area.
- Allow to dry.
- Apply ointment liberally as often as necessary, with each diaper change, especially at bedtime or any time when exposure to wet diapers may be prolonged.
Store at 59° to 86° F (15 ° to 30° C)
Alcohol, Benzethonium Chloride, Cod Liver Oil (contains vitamin A and D), Lavender Fragrance, Mineral Oil, Petrolatum, Sorbitan Monostearate, Talc, Vanillin, and Water.
Protector dérmico
- Ayuda a tratar y prevenir las irritaciones producidas por el pañal.
- Protege la piel irritada debido a las rozaduras de pañal.
- Ayuda a mantener la piel seca.
Para uso externo
*Cuando use este producto no aplicar en los ojos.
Descontinuar su uso y preguntar al médico si:
• la condición empeora
• Los síntomas permanecen por más de 7 días o si desaparecen y vuelven a aparecer luego de pocos días.
No usar en:
• Heridas profundas y punzantes.
• Mordeduras de animales
• Quemaduras graves
* Si son ingeridos, consiga ayuda médica o acuda al Centro de Control de Envenenamiento inmediatamente.
- Cambiar pañales mojados o sucios rápidamente.
- Limpie la zona del pañal.
- Deje secar.
- Aplicar ungüento libremente de acuerdo a necesidad, con cada muda del pañal, especialmente a la hora de dormir o cuando la exposición a pañales mojados se prolongue más de lo habitual.
*Almacenar entre 59° y 86° F (15 y 30°C).
alcohol, cloruro de bencetonio, aceite de hígado de bacalao (contiene vitaminas A y D), esencia de lavanda, aceite mineral, petrolato blanco, monoestearato de sorbitan, talco, vainillina, agua.
Carton Label
Hipoglós® Ointment
Helps prevent and treat diaper rash, skin irritation
Net wt. 4.2 oz (120 g)
| HIPOGLOS
zinc oxide and lanolin ointment |
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
| Labeler - Laboratorios Andromaco S.A. (980106538) |
| Registrant - Laboratorios Andromaco S.A. (980106538) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Laboratorios Andromaco S.A. | 980106538 | manufacture(50145-222) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.