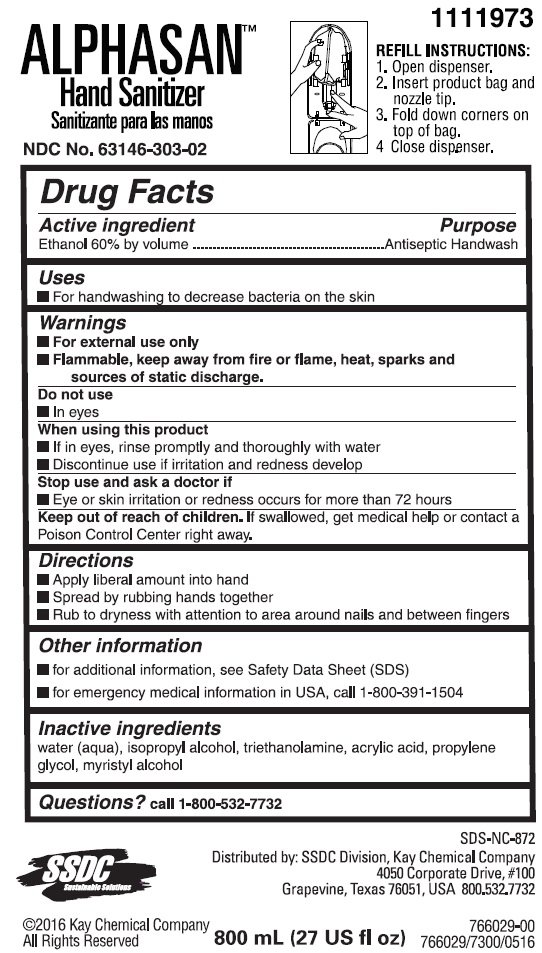
Kay Alphasan Hand Sanitizer
Dosage form: solution
Ingredients: ALCOHOL 60mL in 100mL
Labeler: Kay Chemical Co
NDC code: 63146-303
Medically reviewed by Drugs.com. Last updated on Sep 27, 2023.
Active ingredient
Ethanol 60% by volume
Purpose
Antiseptic Handwash
Uses
- For handwashing to decrease bacteria on the skin
Warnings
- For external use only
-
Flammable, keep away from fire or flame, heat, sparks and sources of static discharge.
Do not use
- In eyes
When using this product
- If in eyes, rinse promptly and thoroughly with water
- Discontinue use if irritation and redness develop
Stop use and ask a doctor if
- Eye or skin irritation or redness occurs for more than 72 hours
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Apply liberal amount into hand
- Spread by rubbing hands together
- Rub to dryness with attention to area around nails and between fingers
Other information
- for additional information, see Material Safety Data Sheet (MSDS)
- for emergency medical information in USA, call 1-800-391-1504
Inactive Ingredients
water (aqua), isopropyl alcohol, triethanolamine, acrylates/C10-30 alkyl acrylate crosspolymer, propylene glycol, myristyl alcohol
Questions? call 1-800-532-7732
| KAY
ALPHASAN HAND SANITIZER
alcohol solution |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - Kay Chemical Co (003237021) |
Document Id: ab84c740-4716-4dd0-bb7a-e9c01b3939e8
Set id: 49180354-18a1-416e-96f0-50ecdeba97ad
Version: 4
Effective Time: 20191007
Kay Chemical Co
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.