par 219 par 219 Pill - beige capsule/oblong, 18mm
Pill with imprint par 219 par 219 is Beige, Capsule/Oblong and has been identified as Doxepin Hydrochloride 50 mg. It is supplied by Par Pharmaceutical Inc.
Doxepin is used in the treatment of Insomnia; Anxiety; Depression; Urticaria; Major Depressive Disorder and belongs to the drug classes miscellaneous anxiolytics, sedatives and hypnotics, tricyclic antidepressants. FDA has not classified the drug for risk during pregnancy. Doxepin 50 mg is not a controlled substance under the Controlled Substances Act (CSA).
Images for par 219 par 219
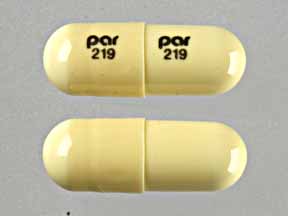
Doxepin Hydrochloride
- Imprint
- par 219 par 219
- Strength
- 50 mg
- Color
- Beige
- Size
- 18.00 mm
- Shape
- Capsule/Oblong
- Availability
- Prescription only
- Drug Class
- Miscellaneous anxiolytics, sedatives and hypnotics, Tricyclic antidepressants
- Pregnancy Category
- N - Not classified
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Par Pharmaceutical Inc.
- National Drug Code (NDC)
- 49884-0219 (Discontinued)
- Inactive Ingredients
-
corn starch,
sodium lauryl sulfate,
magnesium stearate,
D&C Yellow No. 10,
FD&C Yellow No. 6,
titanium dioxide,
gelatin
Note: Inactive ingredients may vary.
Related images for "par 219 par 219"
More about doxepin
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (430)
- Drug images
- Side effects
- Dosage information
- Patient tips
- During pregnancy
- Support group
- Drug class: miscellaneous anxiolytics, sedatives and hypnotics
- Breastfeeding
- En español
Patient resources
- Doxepin capsules, oral concentrate drug information
- Doxepin (Oral) (Advanced Reading)
- Doxepin Tablets
- Doxepin Capsules
- Doxepin Oral Concentrate
Other brands
Professional resources
- Doxepin monograph
- Doxepin (FDA)
- Doxepin Capsules (FDA)
- Doxepin Oral Solution Concentrate (FDA)
- Doxepin Tablets (FDA)
Other brands
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.

