Vitamin B Complex Prescribing Information
Package insert / product label
Dosage form: injection
Drug class: Vitamin and mineral combinations
Medically reviewed by Drugs.com. Last updated on Sep 14, 2023.
On This Page
Vitamin B Complex Description
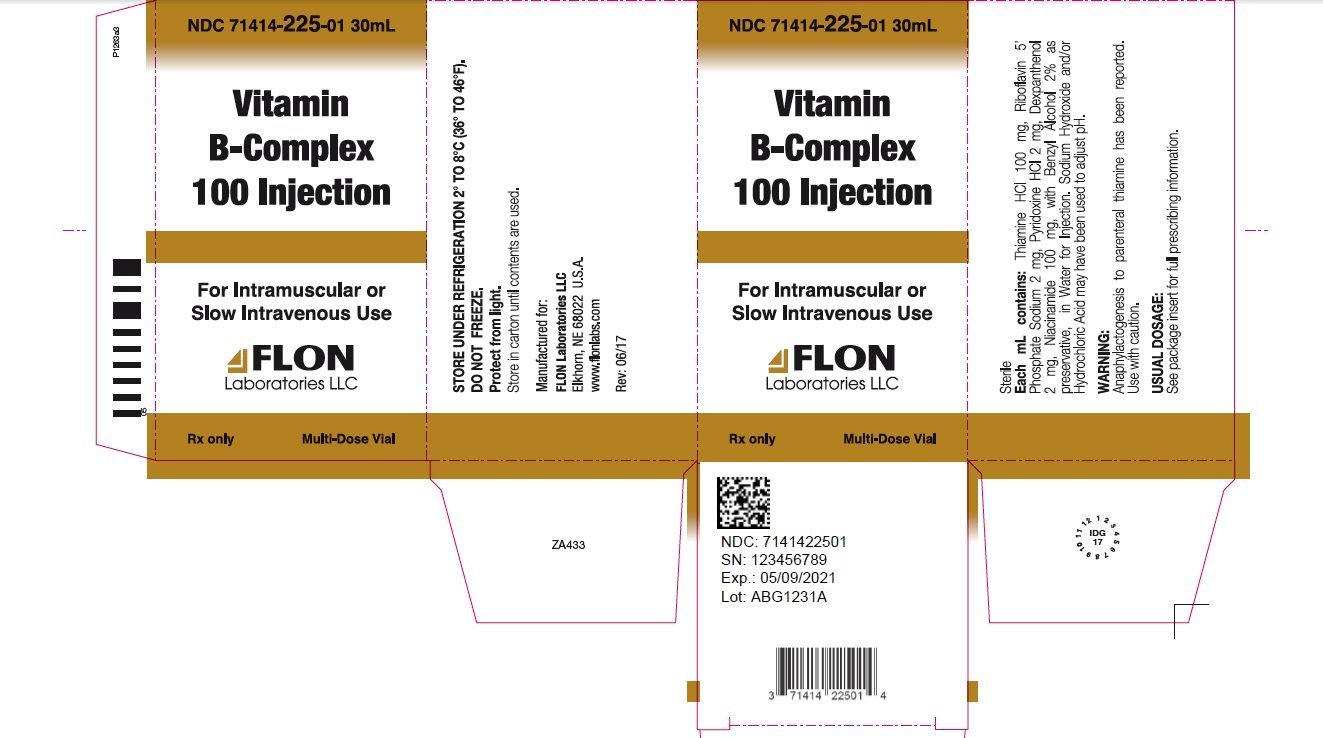
Vitamin B-Complex 100 Injection is a sterile solution for intramuscular or slow intravenous injection comprised of vitamins which may be categorized as belonging to the vitamin B complex group. Each mL contains: Thiamine Hydrochloride 100 mg, Riboflavin 5’ Phosphate Sodium 2 mg, Pyridoxine Hydrochloride 2 mg, Dexpanthenol 2 mg, Niacinamide 100 mg, with Benzyl Alcohol 2% as preservative, in Water for Injection. Sodium Hydroxide and/or Hydrochloric Acid may have been used to adjust pH.
Indications and Usage for Vitamin B Complex
In disorders requiring parenteral administration of vitamins, i.e. pre- and post-operative treatment, when requirements are increased as in fever, severe burns, increased metabolism, pregnancy, gastrointestinal disorders interfering with intake or absorption of vitamins, prolonged or wasting diseases, alcoholism and where other deficiencies exist.
Warnings
Anaphylactogenesis may occur with parenteral thiamine. Use with caution. An intradermal test dose is recommended prior to administration in patients suspected of being sensitive to the drug.
Precautions
The usual precautions for parenteral administration should be observed. Do not inject if precipitation occurs. Inject slowly by the intravenous route. High concentrations should be diluted using Normal Saline Injection when given intravenously.
Adverse Reactions/Side Effects
Mild transient diarrhea, polycythemia vera, peripheral vascular thrombosis, itching transitory exanthema, feeling of swelling of entire body, anaphylactic shock and death. Sensitivity to the ingredients listed may occur (see WARNINGS). Use should be discontinued upon observance of any untoward reaction. Pain upon intramuscular injection may be noted.
Vitamin B Complex Dosage and Administration
Usually 0.25 to 2 mL by intramuscular or slow intravenous injection. High concentrations given intravenously may be diluted using parenteral infusion solutions. (See PRECAUTIONS.)
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever the solution and container permit (see HOW SUPPLIED).
How is Vitamin B Complex supplied
Vitamin B-Complex 100 Injection
NDC 71414-225-01
30 mL Multi-Dose Vial, individually boxed.
Rx Only.
Phase separation due to reduced solubility can occur under certain conditions of shipping or storage (e.g. accidental freezing), which may produce visible particles. Do not use product if these do not redissolve on warming to body temperature and shaking well. Refrigeration of the product may cause darkening of the solution due to the riboflavin content. The colour does not affect the safety or efficacy of the product.
PROTECT FROM LIGHT:
Store in carton until contents are used.
Store under refrigeration 2° to 8°C (36° to 46°F).
Do not permit to freeze.
Manufactured for:
FLON LABORATORIES LLC
Elkhorn, NE 68022 U.S.A
www.flonlabs.com
225PI
REV: 06/17
| VITAMIN B COMPLEX 100
vitamin b complex injection |
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
| Labeler - Fisiopharma SRL (441067444) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Fisiopharma SRL | 441067444 | manufacture(71772-225) | |
Frequently asked questions
More about multivitamin
- Check interactions
- Compare alternatives
- Reviews (232)
- Drug images
- Side effects
- Support group
- Drug class: vitamin and mineral combinations
- En español
Patient resources
Professional resources
- Levomefolate, Calcium Acetylcysteine and Mecobalamin Algal prescribing information
- Lexazin Capsules (FDA)
- Multi Vitamin Infusion M.V.I. 12 (FDA)
- Multi Vitamin Infusion M.V.I. Adult (FDA)
- Multi Vitamin Infusion M.V.I. Pediatric (FDA)
Other brands
Nephplex Rx, Renal Caps, Nephrocaps, MVI Adult, ... +13 more