Sodium Acetate Prescribing Information
Package insert / product label
Dosage form: injection, solution, concentrate
Drug classes: Minerals and electrolytes, Urinary pH modifiers
Medically reviewed by Drugs.com. Last updated on Apr 21, 2024.
On This Page
Sodium Acetate Description
Sodium Acetate Injection, USP 40 mEq (2 mEq/mL) is a sterile, nonpyrogenic, concentrated solution of sodium acetate in water for injection. The solution is administered after dilution by the intravenous route as an electrolyte replenisher. It must not be administered undiluted. Each 20 mL contains 3.28 g of sodium acetate (anhydrous) which provides 40 mEq each of sodium (Na+) and acetate (CH3COO-). The solution contains no bacteriostat, antimicrobial agent or added buffer. May contain acetic acid for pH adjustment; the pH is 6.5 (6.0 to 7.0). The osmolar concentration is 4 mOsmol/mL (calc).
The solution is intended as an alternative to sodium chloride to provide sodium ion (Na+) for addition to large volume infusion fluids for intravenous use.
Sodium Acetate, USP (anhydrous) is chemically designated CH3COONa, a hygroscopic powder very soluble in water.
Sodium Acetate - Clinical Pharmacology
Sodium is the principal cation of extracellular fluid. It comprises more than 90% of total cations at its normal plasma concentration of approximately 140 mEq/liter. The sodium ion exerts a primary role in controlling total body water and its distribution.
Acetate (CH3COO-), a source of hydrogen ion acceptors, is an alternate source of bicarbonate (HCO3-) by metabolic conversion in the liver. This has been shown to proceed readily, even in the presence of severe liver disease.
Related/similar drugs
tolvaptan, urea, ure-Na, Samsca
Indications and Usage for Sodium Acetate
Sodium Acetate Injection, USP 40 mEq is indicated as a source of sodium, for addition to large volume intravenous fluids to prevent or correct hyponatremia in patients with restricted or no oral intake. It is also useful as an additive for preparing specific intravenous fluid formulas when the needs of the patient cannot be met by standard electrolyte or nutrient solutions.
Contraindications
Sodium Acetate Injection, USP 40 mEq is contraindicated in patients with hypernatremia or fluid retention.
Warnings
Sodium Acetate Injection, USP 40 mEq must be diluted before use.
To avoid sodium overload and water retention, infuse sodium-containing solutions slowly.
Solutions containing sodium ions should be used with great care, if at all, in patients with congestive heart failure, severe renal insufficiency and in clinical states in which there exists edema with sodium retention.
In patients with diminished renal function, administration of solutions containing sodium ions may result in sodium retention.
Solutions containing acetate ions should be used with great care in patients with metabolic or respiratory alkalosis. Acetate should be administered with great care in those conditions in which there is an increased level or an impaired utilization of this ion, such as severe hepatic insufficiency.
The intravenous administration of this solution (after appropriate dilution) can cause fluid and/or solute overloading resulting in dilution of other serum electrolyte concentrations, overhydration, congested states or pulmonary edema. Excessive administration of potassium free solutions may result in significant hypokalemia.
WARNING: This product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
Precautions
Do not administer unless solution is clear and seal is intact. Discard unused portion.
Sodium replacement therapy should be guided primarily by the serum sodium level.
Caution should be exercised in administering sodium-containing solutions to patients with severe renal function impairment, cirrhosis, cardiac failure, or other edematous or sodium- retaining states, as well as in patients with oliguria or anuria.
Caution must be exercised in the administration of parenteral fluids, especially those containing sodium ions, to patients receiving corticosteroids or corticotropin.
Solutions containing acetate ions should be used with caution as excess administration may result in metabolic alkalosis.
Pregnancy: Animal reproduction studies have not been conducted with sodium acetate. It is also not known whether sodium acetate can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Sodium Acetate should be given to a pregnant woman only if clearly needed.
Pediatric Use: Safety and effectiveness have been established in the age groups infant to adolescent.
Geriatric Use: An evaluation of current literature revealed no clinical experience identifying differences in response between elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Sodium ions are known to be substantially excreted by the kidney, and the risk of toxic reactions may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Adverse Reactions/Side Effects
Sodium overload can occur with intravenous infusion of excessive amounts of sodium- containing solutions. See WARNINGS and PRECAUTIONS.
Overdosage
In the event of overdosage, discontinue infusion containing sodium acetate immediately and institute corrective therapy as indicated to reduce elevated serum sodium levels, and restore acid- base balance if necessary. See WARNINGS, PRECAUTIONS and ADVERSE REACTIONS.
Sodium Acetate Dosage and Administration
Sodium Acetate Injection, USP 40 mEq is administered intravenously only after dilution in a larger volume of fluid. The dose and rate of administration are dependent upon the individual needs of the patient. Serum sodium should be monitored as a guide to dosage. Using aseptic technique, all or part of the contents of one or more vials may be added to other intravenous fluids to provide any desired number of milliequivalents (mEq) of sodium (Na+) with an equal number of acetate (CH3COO-).
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. See PRECAUTIONS.
How is Sodium Acetate supplied
Sodium Acetate Injection, USP 40 mEq is supplied as follows:
|
Total Amounts |
||||
|
NDC No. |
Fill Volume |
Na+ |
Acetate |
Concentration |
|
69784-229-10 |
20 mL |
40 mEq |
40 mEq |
16.4% |
Sodium Acetate Injection, USP 40 mEq per 20 mL vials are suppled as 10 vials per carton, with a single package insert.
Each vial is partially filled to provide air space for complete vacuum withdrawal of the contents into the intravenous container.
Store at 20° to 25ºC (68° to 77ºF). [See USP Controlled Room Temperature.]
ALSO AVAILABLE AS
Sodium Acetate Injection, USP is also supplied in Pharmacy Bulk Packages as follows:
| NDC No. | Fill Volume | Na+ | Acetate | Concentration |
| 69784-230-10 | 50 mL | 100 mEq | 100 mEq | 16.4% |
| 69784-231-10 | 100 mL | 200 mEq | 200 mEq | 16.4% |
|
69784-232-10 – Glass 69784-232-01 – Case | 100 mL | 400 mEq | 400 mEq | 32.8% |
Revised: Feb 2024
Manufactured by:
S.M. Farmaceutici SRL
Tito – 85050, Italy
Distributed by:
Woodward Pharma Services LLC
Wixom, MI 48393
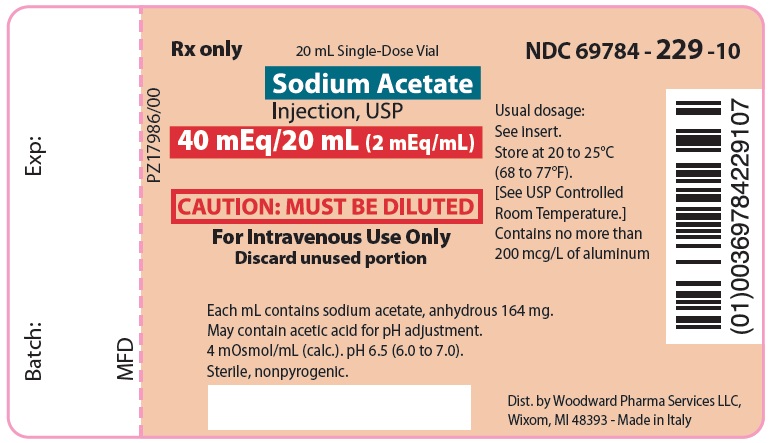
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Sodium Acetate
Injection, USP
40 mEq/20 mL (2 mEq/mL)
CAUTION: MUST BE DILUTED
For Intravenous Use Only
NDC 69784-229-10 Rx only
Each mL contains sodium acetate, anhydrous 164 mg. May contain acetic acid for pH adjustment. 4 mOsmol/mL (calc.). pH 6.5 (6.0 to 7.0).
Sterile, nonpyrogenic.
Usual dosage: See insert.
Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.]
Contains no more than 200 mcg/L of aluminum.
Dist. by Woodward Pharma Services LLC, Wixom, MI 48393 - Made in Italy
Sodium Acetate
Injection, USP
40 mEq/20 mL (2 mEq/mL)
CAUTION: MUST BE DILUTED
For Intravenous Use Only
NDC 69784-229-10 Rx only
Each mL contains sodium acetate, anhydrous 164 mg. May contain acetic acid for pH adjustment. 4 mOsmol/mL (calc.). pH 6.5 (6.0 to 7.0).
Sterile, nonpyrogenic.
USE ASEPTIC TECHNIQUE
Remove cover from the Fliptop Vial and cleanse stopper with antiseptic. Contains no bacteriostat; use promptly; discard unused portion.
Use only if clear and seal is intact and undamaged.
Usual dosage: See insert.
Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.]
Contains no more than 200 mcg/L of aluminum.
Dist. by Woodward Pharma Services LLC, Wixom, MI 48393 - Made in Italy

| SODIUM ACETATE
sodium acetate injection, solution, concentrate |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - WOODWARD PHARMA SERVICES LLC (080406260) |
| Registrant - MILLA PHARMACEUTICALS, INC. (080563277) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| S.M. FARMACEUTICI SRL | 430188286 | manufacture(69784-229) | |
More about sodium acetate
- Check interactions
- Compare alternatives
- Pricing & coupons
- Latest FDA alerts (1)
- Side effects
- Dosage information
- During pregnancy
- Drug class: minerals and electrolytes
