Naftifine Gel Prescribing Information
Package insert / product label
Generic name: naftifine hydrochloride
Dosage form: gel
Drug class: Topical antifungals
Medically reviewed by Drugs.com. Last updated on Feb 7, 2024.
On This Page
Naftifine Gel Description
Naftifine Hydrochloride Gel USP, 1% contains the synthetic, broad-spectrum, antifungal agent naftifine hydrochloride.
Naftifine Hydrochloride Gel USP, 1% is for topical use only.
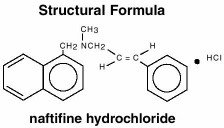
Naftifine Hydrochloride Chemical Structure
Chemical Name
(E)-N-Cinnamyl-N-methyl-1-naphthalenemethylamine hydrochloride.
Naftifine hydrochloride has an empirical formula of C21H21N•HCl and a molecular weight of 323.86.
Contains
Active Ingredient
Naftifine hydrochloride…. 1%
Inactive Ingredients
Alcohol (52%v/v), carbopol 974P, diisopropanolamine, edetate disodium, polysorbate 80 and purified water.
Naftifine Gel - Clinical Pharmacology
Naftifine hydrochloride is a synthetic allylamine derivative. The following in vitro data are available, but their clinical significance is unknown. Naftifine hydrochloride has been shown to exhibit fungicidal activity in vitro against a broad spectrum of organisms, including Trichophyton rubrum, Trichophyton mentagrophytes, Trichophyton tonsurans, Epidermophyton floccosum, Microsporum canis, Microsporum audouini, and Microsporum gypseum; and fungistatic activity against Candida species, including Candida albicans. Naftifine Hydrochloride Gel, 1% has only been shown to be clinically effective against the disease entities listed in the INDICATIONS AND USAGE section.
Although the exact mechanism of action against fungi is not known, naftifine hydrochloride appears to interfere with sterol biosynthesis by inhibiting the enzyme squalene 2, 3-epoxidase. This inhibition of enzyme activity results in decreased amounts of sterols, especially ergosterol, and a corresponding accumulation of squalene in the cells.
Pharmacokinetics
In vitro and in vivo bioavailability studies have demonstrated that naftifine penetrates the stratum corneum in sufficient concentration to inhibit the growth of dermatophytes.
Following single topical applications of 3H-labeled Naftifine Hydrochloride Gel, 1% to the skin of healthy subjects, up to 4.2% of the applied dose was absorbed. Naftifine and/or its metabolites are excreted via the urine and feces with a half-life of approximately two to three days.
Related/similar drugs
clotrimazole topical, ketoconazole topical, terbinafine, miconazole topical, Lamisil
Indications and Usage for Naftifine Gel
Naftifine Hydrochloride Gel USP, 1% is indicated for the topical treatment of tinea pedis, tinea cruris and tinea corporis caused by the organisms Trichophyton rubrum, Trichophyton mentagrophytes, Trichophyton tonsurans1 and Epidermophyton floccosum.1
1Efficacy for this organism in this organ system was studied in fewer than 10 infections.
Contraindications
Naftifine Hydrochloride Gel, 1% is contraindicated in individuals who have shown hypersensitivity to any of its components.
Precautions
General
Naftifine Hydrochloride Gel, 1% is for external use only. If irritation or sensitivity develops with the use of Naftifine Hydrochloride Gel, 1%, treatment should be discontinued and appropriate therapy instituted. Diagnosis of the disease should be confirmed either by direct microscopic examination of a mounting of infected tissue in a solution of potassium hydroxide or by culture on an appropriate medium.
Information for patients
The patient should be told to:
- Avoid the use of occlusive dressings or wrappings unless otherwise directed by the physician.
- Keep Naftifine Hydrochloride Gel, 1% away from the eyes, nose, mouth and other mucous membranes.
Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 2-year dermal carcinogenicity study, naftifine hydrochloride cream was administered to Sprague-Dawley rats at topical doses of 1%, 2% and 3% (10, 20, and 30 mg/kg/day naftifine hydrochloride). No drug-related tumors were noted in this study up to the highest dose evaluated in this study of 30 mg/kg/day [3.6 times the maximum recommended human dose (MRHD) based on mg/m2 comparison].
Naftifine hydrochloride revealed no evidence of mutagenic or clastogenic potential based on the results of two in vitro genotoxicity tests (Ames assay and Chinese hamster ovary cell chromosome aberration assay) and one in vivo genotoxicity test (mouse bone marrow micronucleus assay).
Oral administration of naftifine hydrochloride to rats, throughout mating, gestation, parturition and lactation, demonstrated no effects on growth, fertility or reproduction, at doses up to 100 mg/kg/day (12 times MRHD based on mg/m2 comparison).
Pregnancy
Teratogenic Effects
Reproduction studies have been performed in rats and rabbits (via oral administration) at doses 150 times or more than the topical human dose and have revealed no evidence of impaired fertility or harm to the fetus due to naftifine. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Adverse Reactions/Side Effects
During clinical trials with Naftifine Hydrochloride Gel, 1% the incidence of adverse reactions was as follows: burning/stinging (5.0%), itching (1.0%), erythema (0.5%), rash (0.5%), skin tenderness (0.5%).
Naftifine Gel Dosage and Administration
A sufficient quantity of Naftifine Hydrochloride Gel USP, 1% should be gently massaged into the affected and surrounding skin areas twice a day, in the morning and evening. The hands should be washed after application. If no clinical improvement is seen after four weeks of treatment with Naftifine Hydrochloride Gel USP, 1% the patient should be re-evaluated.
How is Naftifine Gel supplied
Naftifine Hydrochloride Gel USP, 1% is supplied in collapsible tubes in the following sizes:
40g – NDC 0115-1510-63
60g – NDC 0115-1510-58
90g – NDC 0115-1510-48
Note: Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
To report SUSPECTED ADVERSE REACTIONS contact Amneal Pharmaceuticals at 1-877-835-5472 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Manufactured by:
Tolmar, Inc.
Fort Collins, CO 80526
Distributed by:
Amneal Pharmaceuticals LLC
Bridgewater, NJ 08807
04006119 Rev. 0 11/18
| NAFTIFINE HYDROCHLORIDE
naftifine hydrochloride gel |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Amneal Pharmaceuticals of New York LLC (123797875) |
More about naftifine topical
- Compare alternatives
- Pricing & coupons
- Reviews (7)
- Side effects
- Dosage information
- During pregnancy
- Drug class: topical antifungals
- Breastfeeding
- En español

