Quflora Pediatric Prescribing Information
Package insert / product label
Generic name: vitamin a acetate, ascorbic acid, cholecalciferol, .alpha.-tocopherol, dl-, thiamine hydrochloride, riboflavin, niacinamide, pyridoxine hydrochloride, levomefolic acid, folic acid, cyanocobalamin, magnesium oxide, cupric sulfate and sodium fluoride
Dosage form: tablet, chewable
Drug class: Vitamin and mineral combinations
Medically reviewed by Drugs.com. Last updated on Jan 16, 2024.
On This Page
| Supplement Facts | ||
|---|---|---|
| Serving Size: 1 Tablet Servings per Container: 30 |
||
| Amount per Serving | % Daily Value* |
|
| Vitamin A (as Acetate) | 1,200 IU | 24% |
| Vitamin C (as Ascorbic Acid) | 60 mg | 100% |
| Vitamin D3 (as Cholecalciferol) | 400 IU | 100% |
| Vitamin E (as DL-Alpha Tocopheryl Acetate) | 15 IU | 50% |
| Thiamin (as Thiamine HCl, Vitamin B1) | 1.2 mg | 80% |
| Riboflavin (Vitamin B2) | 1.3 mg | 76% |
| Niacin (as Niacinamide) | 5 mg | 25% |
| Vitamin B6 (as Pyridoxine HCl) | 1.5 mg | 75% |
| Folate (as 200 mcg Quatrefolic® ((6S-5-methyltetrahydrofolic acid, glucosamine salt, molar equivalent to 108 mcg of Folic Acid), and 100 mcg of folic acid) | 208 mcg | 52% |
| Vitamin B12 (as Cyanocobalamin) | 4 mcg | 67% |
| Magnesium (as Magnesium Oxide) | 15 mg | 4% |
| Copper (as Cupric Sulfate) | 1 mg | 50% |
| Fluoride (as Sodium Fluoride) | 0.5 mg | † |
Other Ingredients: Citric acid, fumed silica, magnesium stearate, microcrystalline cellulose, natural grape flavor, silicon dioxide, stearic acid, sucralose, sucrose and xylitol.
Indications and Usage for Quflora Pediatric
Quflora™ Pediatric Chewable Tablets with 0.5 mg Fluoride is a prescription dietary fluoride supplement providing twelve essential vitamins and minerals.
Contraindications
Quflora™ Pediatric Chewable Tablets with 0.5 mg Fluoride should not be used by patients with a known history of hypersensitivity to any of the listed ingredients.
Precautions
General
The suggested dose should not be exceeded, since dental fluorosis may result from continued ingestion of large amounts of fluoride. Do not eat or drink dairy products within one hour of fluoride administration. Incompatibility of fluoride with dairy foods has been reported due to formation of calcium fluoride which is poorly absorbed. Your healthcare practitioner can prescribe the correct dosage.
Folic Acid
Folic Acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress.
Adverse Reactions/Side Effects
Allergic rash and other idiosyncrasies have been rarely reported. Call your doctor for medical advice about side effects. You may report side effects or obtain product information by calling Carwin Pharmaceutical Associates, LLC at 1-844-700-5011.
Related/similar drugs
ferrous sulfate, ergocalciferol, folic acid, thiamine, Zinc, FeroSul
Warnings
Keep out of the reach of children. In case of accidental overdose, seek professional emergency assistance or contact a Poison Control Center immediately. This product should be chewed and is not recommended for children under age 4.
Quflora Pediatric Dosage and Administration
One tablet daily or as prescribed by your healthcare practitioner. Each tablet contains 0.5 mg fluoride ion (F) from 1.1 mg sodium fluoride (NaF).
Quflora Pediatric Description
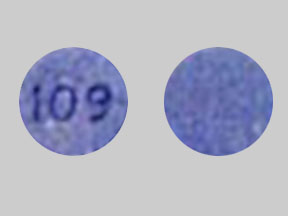
Quflora™ Pediatric Chewable Tablets with 0.5 mg Fluoride is a yellow round tablet imprinted "104".
How is Quflora Pediatric supplied
Quflora™ Pediatric Chewable Tablets with 0.5 mg Fluoride available in child-resistant bottles of 30 (Product Code: 15370-104-30).
Rx Only
Quatrefolic® is a registered trademark of Gnosis, SpA. Covered by one or more claims of U.S. Patent # 7,947,662 CAS# 1181972-37-1
Manufactured for:
Carwin Pharmaceutical Associates, LLC
Hazlet, NJ 07730
www.carwinpharma.com
Made in Canada
Rev. 4/16
PRINCIPAL DISPLAY PANEL - 0.25 mg Tablet Bottle Label
15370-103-30
QUfLORA™
PEDIATRIC
Chewable Tablets
Multivitamin with
0.25 mg Fluoride
Each chewable tablet contains:
0.55 mg sodium fluoride, USP
Rx Only • Grape Flavor
30 Tablets
carwin
PHARMACEUTICAL ASSOCIATES

| QUFLORA PEDIATRIC
vitamin a acetate, ascorbic acid, cholecalciferol, .alpha.-tocopherol, dl-, thiamine hydrochloride, riboflavin, niacinamide, pyridoxine hydrochloride, levomefolic acid, folic acid, cyanocobalamin, magnesium oxide, cupric sulfate, and sodium fluoride tablet, chewable |
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| QUFLORA PEDIATRIC
vitamin a acetate, ascorbic acid, cholecalciferol, .alpha.-tocopherol, dl-, thiamine hydrochloride, riboflavin, niacinamide, pyridoxine hydrochloride, levomefolic acid, folic acid, cyanocobalamin, magnesium oxide, cupric sulfate, and sodium fluoride tablet, chewable |
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| QUFLORA PEDIATRIC
vitamin a acetate, ascorbic acid, cholecalciferol, .alpha.-tocopherol, dl-, thiamine hydrochloride, riboflavin, niacinamide, pyridoxine hydrochloride, levomefolic acid, folic acid, cyanocobalamin, magnesium oxide, cupric sulfate, and sodium fluoride tablet, chewable |
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - CarWin Pharmaceutical Associates, LLC (079217215) |
More about multivitamin with fluoride
- Check interactions
- Compare alternatives
- Pricing & coupons
- Drug images
- Side effects
- Dosage information
- Drug class: vitamin and mineral combinations
- En español
Patient resources
- Multivitamins with fluoride drug information
- Pediatric Multivitamin Chewables with Fluoride
- Pediatric Multivitamin Drops with Fluoride
Professional resources
- Multi Vitamin Fluoride Drops prescribing information
- Multi Vitamin with Fluoride (FDA)
- Multi-Vit with Fluoride Drops (FDA)
- MultiVit with Fluoride Chewable Tablets (FDA)
- Multivitamin with Fluoride Chewable Tablets (FDA)
- Vitamins A, C, D and Fluoride (FDA)
Other brands
MVC-Fluoride, TRI-VIT With Fluoride, Tri-Vite Drops with Fluoride